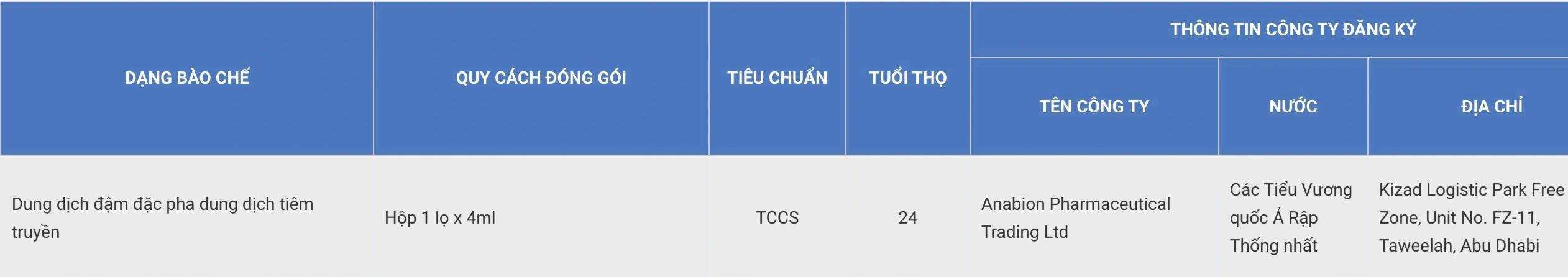
In the list licensed by the Drug Administration, Pembroria (Pembrolizumab active ingredient, content 100mg/4ml) - a cancer treatment drug manufactured by Liability Limited "PK-137" (Russia Federation) and Anabion Pharmaceutical Trading Ltd (united Arab Emirates) is registered for circulation.
The drug is prepared in the form of a special solution for injection, with a shelf life of 24 months from the date of manufacture.
For Pembroria, the Drug Administration requires the company to update the clinical trial progress for phase III every 3 months and supplement data after completing the study.
Before being licensed for circulation in Vietnam, the Pembroria drug dossier was assessed by experts from Hanoi Medical University, confirming that it met the requirements for effectiveness and immunocompromisation similar to starting foods.
According to the Department of Drug Administration, the drug is a single-line antibodies that have completed clinical trials. This is a treatment, not a vaccine, and is not a new invention, because Vietnam has been circulating the original drug of the same type since 2017.
To date, the Drug Administration of Vietnam has licensed 99 drugs and single antibodies for cancer treatment, including Pembroria.
destination drugs for cancer treatment have been used in Vietnam for many years. The newly licensed Russian drug is a similar biological product, opening up more treatment options for patients.
Pembroria drug costs about 18 million VND per bottle, and patients often need 2 bottles for one treatment. The treatment can last from 12 to 24 sessions, until the drug is no longer effective.
Currently, this drug is not on the list of drugs covered by health insurance.











