Every year, the disease begins to appear from the end of December and increases sharply in the first months of the year, with a peak from February to May - the time when children participate in many group activities and crowded environments.
According to statistics from the Pasteur Institute of Ho Chi Minh City, 90% of patients infected with chickenpox are children between the ages of 2 and 7.
Because this is an contagious disease, children who go to school and are often exposed to crowded environments are at higher risk of disease.
Bean sprouts can cause complications, especially when children have weak resistance or improper care:
- Skin infections, impetigo, infections caused by bacteria.
- viral pneumonia
- Encephalitis, triceratitis
- Dehydration when children have a high fever, belly, stop drinking
In the long term, children may experience complications such as being susceptible to shingles, also known as scabies, a late recurrence of chickenpox after many years. zona also has other complications such as: neuropathic pain, corneal ulcers, blindness...
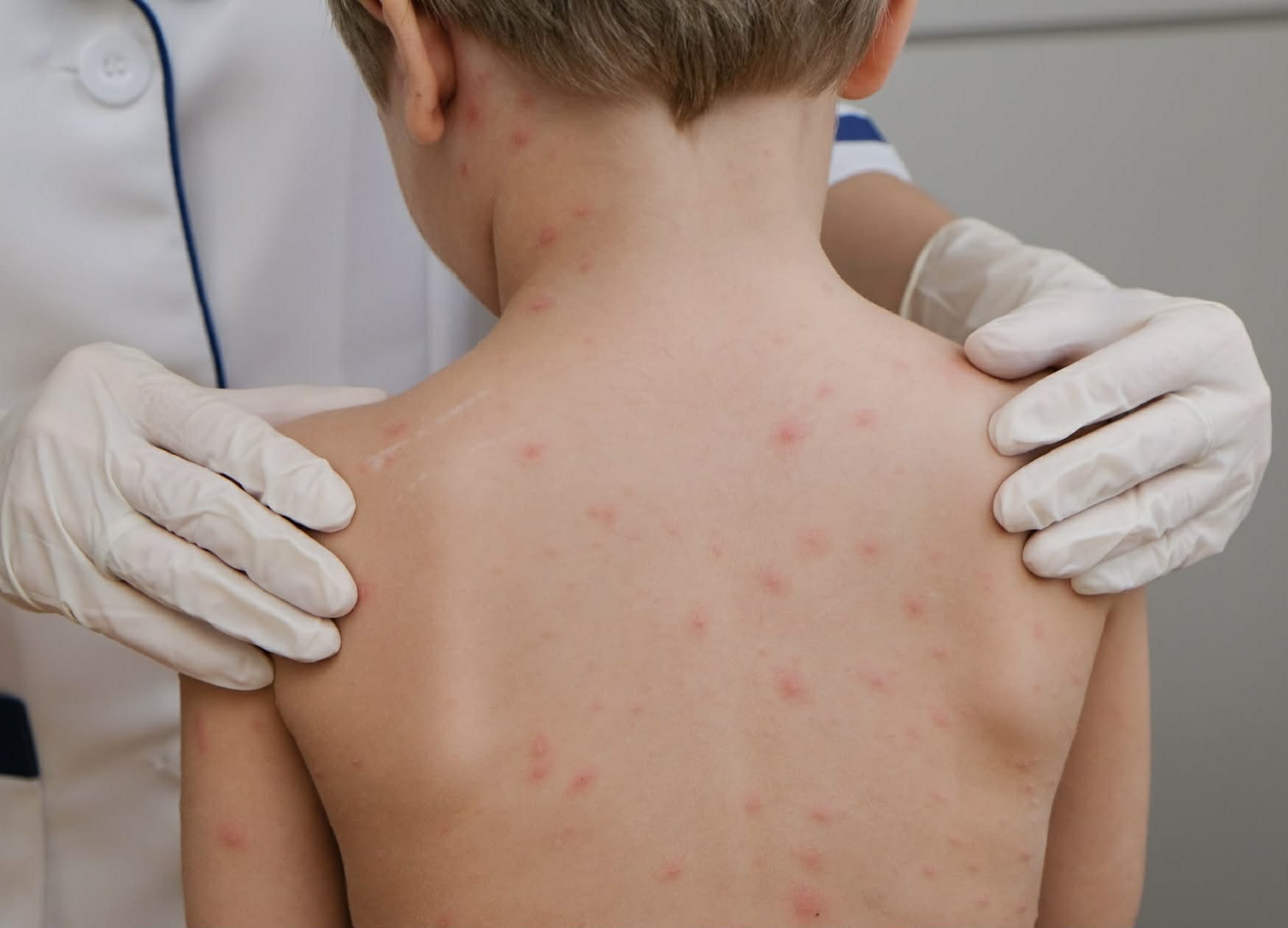
Health experts recommend that vaccination is the most proactive, safe and effective method of preventing chickenpox today. Vaccination helps reduce the risk of disease, limit complications and protect children throughout the epidemic season. Children aged 12 months and over are recommended to get vaccinated early to create active immunity, helping to reduce the risk of disease and complications.
Currently, the chickenpox vaccine has many improvements, such as the 2nd generation of the MAV/06 chickenpox vaccine, produced by GC Biopharma (Korea) with outstanding advantages such as:
- High and stable immunity, minimum effectiveness ≥ 3800 PFU
- Do not contain antibiotics in ingredients, so reduce the risk of sensitivity in children with allergic rhinitis.
- Achieved WHO Prequalification (WHO-PQ) for quality and safety, recommended by experts to be vaccinated early for children to protect long-term health.
- Reasonable price, support for expanding vaccination coverage.
Barycela has a reasonable cost, making it easier for vaccination facilities to deploy services for many population groups. Reasonable prices help parents get their children vaccinated earlier, thereby increasing vaccine coverage, reducing the risk of outbreaks and reducing the burden of treatment in the community.
Recommended vaccination schedule
Children 12 months - 12 years old:
- Snoring 1: from 12 months old
- Nose 2: After nose 1, at least 3 months or get a Tet injection when the child is 46 years old.
Early chicken vaccination is an immune shield that helps children stay healthy, enjoy their childhood and protect them from the risk of long-term illness.
NAV VAT GROUP is a unit authorized to distribute the second generation of the MAV 06 chickenpox vaccine, called BarYCELA inj. (GC Biopharma - Korea) in Vietnam. With more than 21 years of experience in the field of vaccines and health products, NAVIVA GROUP cooperates with medical facilities to enhance access to safe - effective - quality vaccines for the community




