Since the beginning of 2025, the Center for Allergy & Clinical Immunology (Bach Mai Hospital) has received many severe drug allergy cases related to Phenylbutazone - an anti-inflammatory drug banned by the Ministry of Health since 2013. Among them, there are patients who have died from serious complications.
Four typical cases have just been recorded. A 51-year-old male patient in Hai Phong bought Phenylbutazone online to treat joint pain. After a short time of use, the patient had a high fever, a full-body rash and was diagnosed with DRESS syndrome - a dangerous drug allergy reaction that can cause multiple organ damage.
Another case was a 66-year-old male patient in Nghe An who was given Phenylbutazone continuously by his family for 35 days. The patient had a dengue redness, accompanied by fever and was diagnosed with DRESS syndrome.
The next case was a 39-year-old male patient in Phu Tho who took Phenylbutazone purchased online for 30 days. After that, the patient had a fever, sore throat, a whole body rash and was diagnosed with DRESS syndrome.
A 70-year-old female patient in Phu Tho arbitrarily used Phenylbutazone in combination with drugs of unknown origin. The patient had a high fever, mouth ulcers, eye pain, acute liver failure, quickly fell into septic shock and died. Final diagnosis: Lyell syndrome (poisoned skin necrosis) - a rare complication but has a high mortality rate.
Dr. Chu Chi Hieu - Head of the Department of Duodenal Allergics, Center for Clinical Allergy & Immunology, Bach Mai Hospital - said: Phenylbutazone - a non-steroidal anti-inflammatory drug (NSAID) - has been banned by the Ministry of Health since 2013 due to the risk of causing serious allergies, threatening life. Drugs can cause DRESS syndrome (high fever, rash, liver, kidney, heart, lung damage), Lyell syndrome (wide skin peeling, multiple organ failure, high mortality), along with bone marrow failure, digestive bleeding, acute kidney failure. The risk of complications does not depend on the dosage or duration of use, so even short-term treatment has potential risks. The purchase and use of this drug is strictly prohibited and will be handled according to the law.
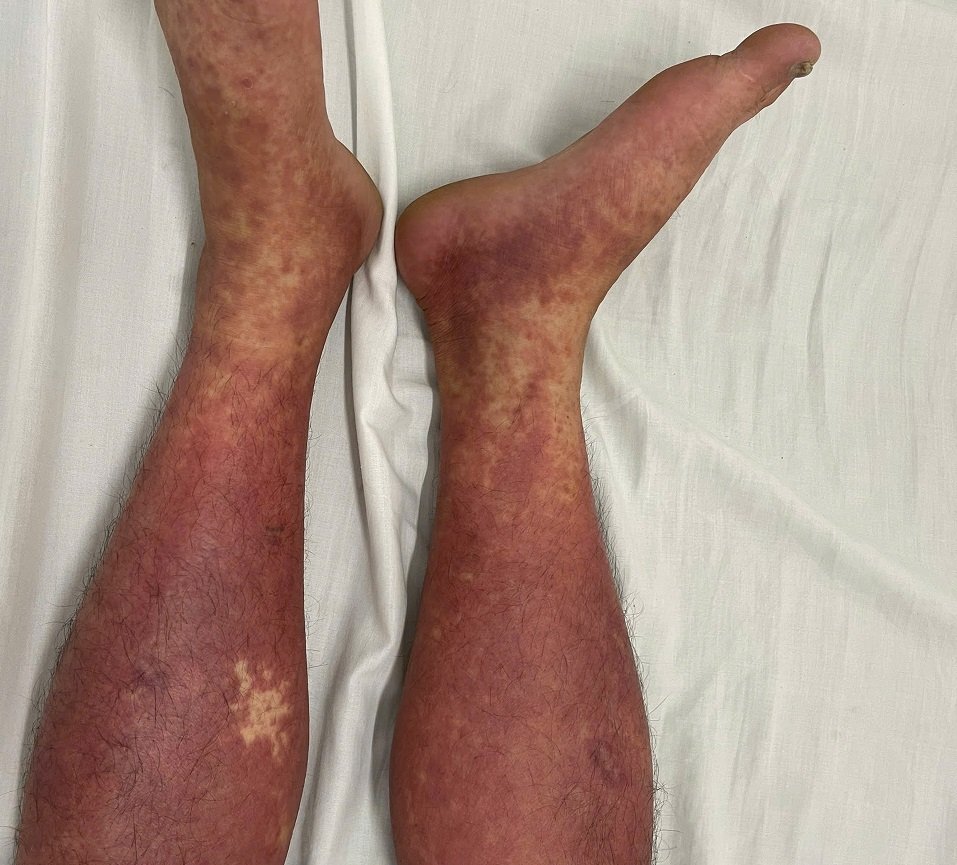
"The common point in these cases is the time of late symptoms - at least 1 week after starting to take the drug, in some cases even 13 months before showing unusual signs. This causes many difficulties in exploiting the history to find the cause of the allergy, especially when the patient cannot remember the exact name of the drug or uses a pharmacy in foreign languages.
In addition, initial symptoms such as fever, rash, and fatigue are easily confused with common infectious diseases, causing patients to be examined and treated in other specialties, slowing down the diagnosis and timely intervention process," said Dr. Chu Chi Hieu.











