The Ministry of Health signed and issued Circular No. 26/2025/TT-BYT dated June 30, 2025 regulating prescriptions for drugs and prescriptions for pharmaceutical and biological products in outpatient treatment at medical examination and treatment facilities. The Circular takes effect from July 1, marking an important change to facilitate patients.
According to the new regulation, people with some chronic diseases on the permitted list will be prescribed outpatient medicines for more than 30 days, up to 90 days, instead of being only given medicines for up to 30 days as before according to Circular 52/2017.
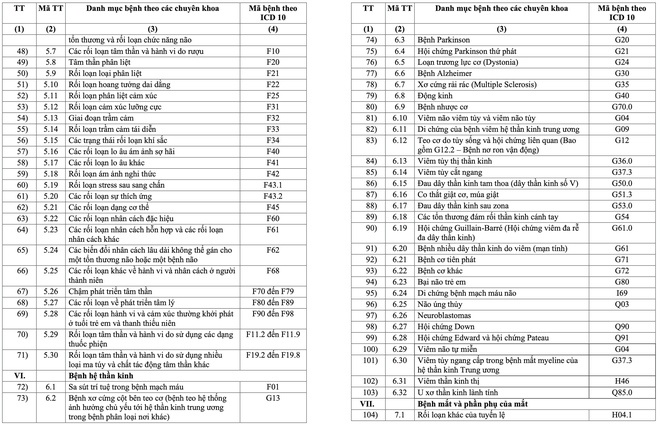
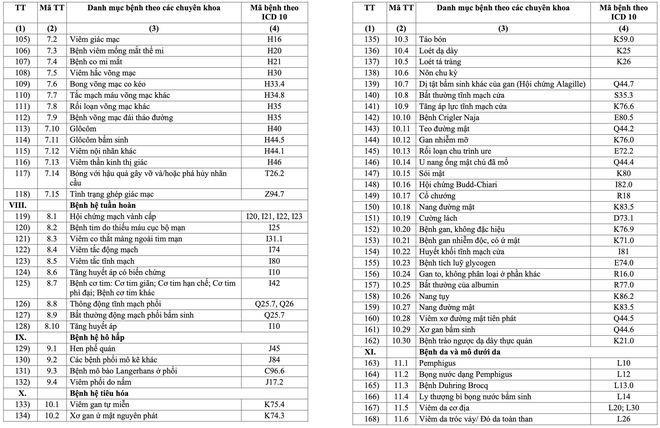
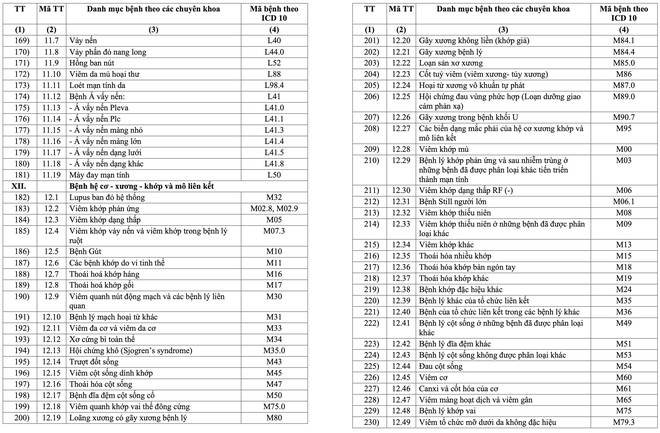
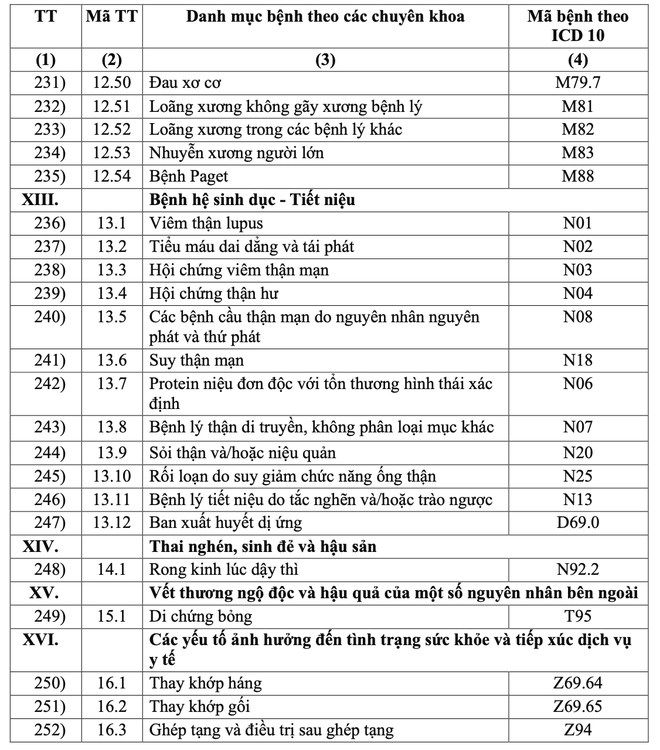
The list includes 16 major groups of diseases, with a total of 252 diseases and groups of chronic diseases. These include many common diseases such as high blood pressure, diabetes, COPD, bronchitis, chronic hepatitis B, HIV/AIDS, Parkinson's, Alzheimer's, depression, anxiety disorder, congenital hemodystasis ( Thalassemia), hypothyroidism, lai offside, dementia and gynecological diseases in adolescence such as menopause. Some cancers such as breast cancer and thyroid cancer are also subject to long-term medication.