The Department of Drug Administration (Ministry of Health) has just issued a decision to revoke all cosmetic product declaration forms and suspend the circulation of products of MK Skincare Company distributed by Mrs. Phan Thi Mai's husband (Mailisa).
According to the Department of Drug Administration, MK Skincare Company had all of its declaration forms revoked for not presenting product information documents (PIF) according to the regulations of the Ministry of Health.
Not only stopping at revoking the declaration form, the Drug Administration Department also suspended circulation and nationwide revoked all expired batches of 162 cosmetic products launched by MK Skincare Company.
According to the Department of Drug Administration, MK Skincare Company is responsible for sending a notice of revocation to the places distributing and using the above product lots; receiving products returned from business establishments, recalling product lots, special-storage and preserving the recalled cosmetic products until there is a opinion on the destruction by the competent state agency.
At the same time, report the revocation of the above cosmetic products to the Department of Drug Administration before December 15, 2025.
Here are details of 162 products of Mailisa beauty salon that have been recalled nationwide:

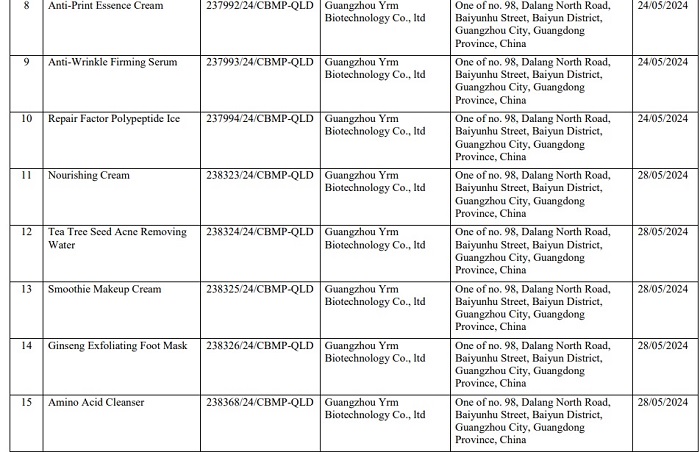
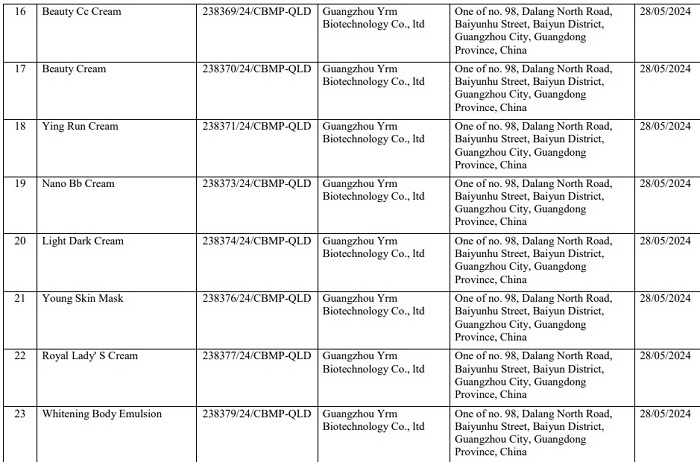
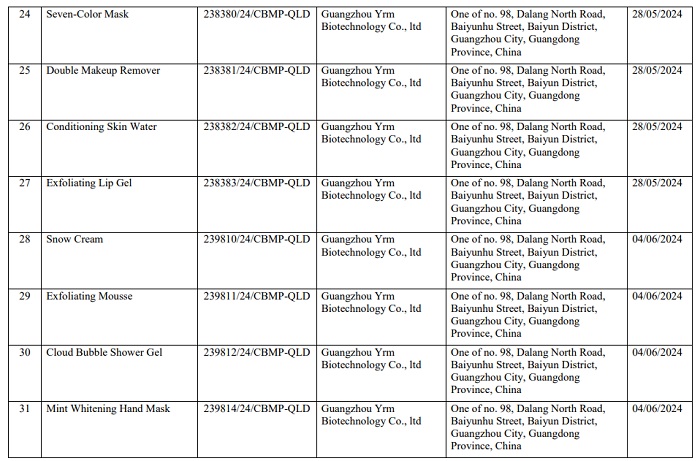
Readers can see detailed product lists of Mailisa products that have been revoked HERE.
Previously, the Department of Investigation Police on Corruption, Economic Crimes, and Smuggling (C03 - Ministry of Public Security) continued to investigate violations related to MK Skincare Import-Export Production and Trading Service Company Limited and the Mailisa beauty salon system.
According to initial investigation results, cosmetic products in the Mailisa ecosystem - including Doctor Magic products - were determined to originate from Guangzhou (China), belonging to the group of cheap goods and not meeting the standards and ingredients to be granted a free circulation certificate (CFS) in China.
The investigation agency accused Ms. Phan Thi Mai and Mr. Hoang Kim Khanh - the owner of the Mailisa beauty salon system - of colluding with Chinese partners to make "forged" contracts, converting the origin from Guangzhou to Hong Kong (China). This is to legalize the CFS application before the goods are imported to Vietnam by MK Skincare Company - operated by Mr. Khanh - for distribution. Initial estimates show that with only 3 key products out of about 100 products of the system, the amount of illegal profits could reach thousands of billions of VND.
The main products of Mailisa beauty salon include the N3 set - a set of moisturizer to help improve melasma, velvet, and prey hill for sale at 2.3 million VND; the Doctor Magic Melasma leather combo (N3+M23) for sale at 2.74 million VND; the B1 set - a set of moisturizer to help brighten the skin for sale at 1.7 million VND.
At the investigation agency, Hoang Kim Khanh stated that he ordered cosmetics from Guangzhou (China) but changed the origin to "Hong Kong - China" due to concerns that the market would not favor Chinese goods and products without CFS. Because he arbitrarily changed his origin, Khanh had to transfer money through Ms. Do Bich Thuy to pay the partner. Khanh admitted to using a fake contract to bring cosmetics with Hong Kong (China) labels to Vietnam.











