On November 25, the Department of Drug Administration (Ministry of Health) revoked 80 receipt numbers of declarations and suspended the circulation of 162 cosmetic products issued by MK Skincare Import-Export Production and Trading Service Company Limited - a company owned by Mr. Hoang Kim Khanh, Ms. Mailisa's husband - responsible for launching them on the market.
The reason is that the company did not present the Product Information Document (PIF) as prescribed, mandatory documents to prove the safety, quality and effectiveness of the product, causing these products to pose a potential risk to the safety of users.
All expired batches of 162 products in the recalled portfolio - mostly manufactured at multiple facilities in China - have been suspended from circulation nationwide. This decision is applied as a remedial measure under Article 71 of Decree 117/2020/ND-CP to ensure the safety of users when the enterprise cannot provide PIF documents as prescribed.
On November 26, the official website of Mailisa beauty salon was still active but the "Product" section was removed. However, when typing to search for the names of Doctor Magic products online, they still appear.
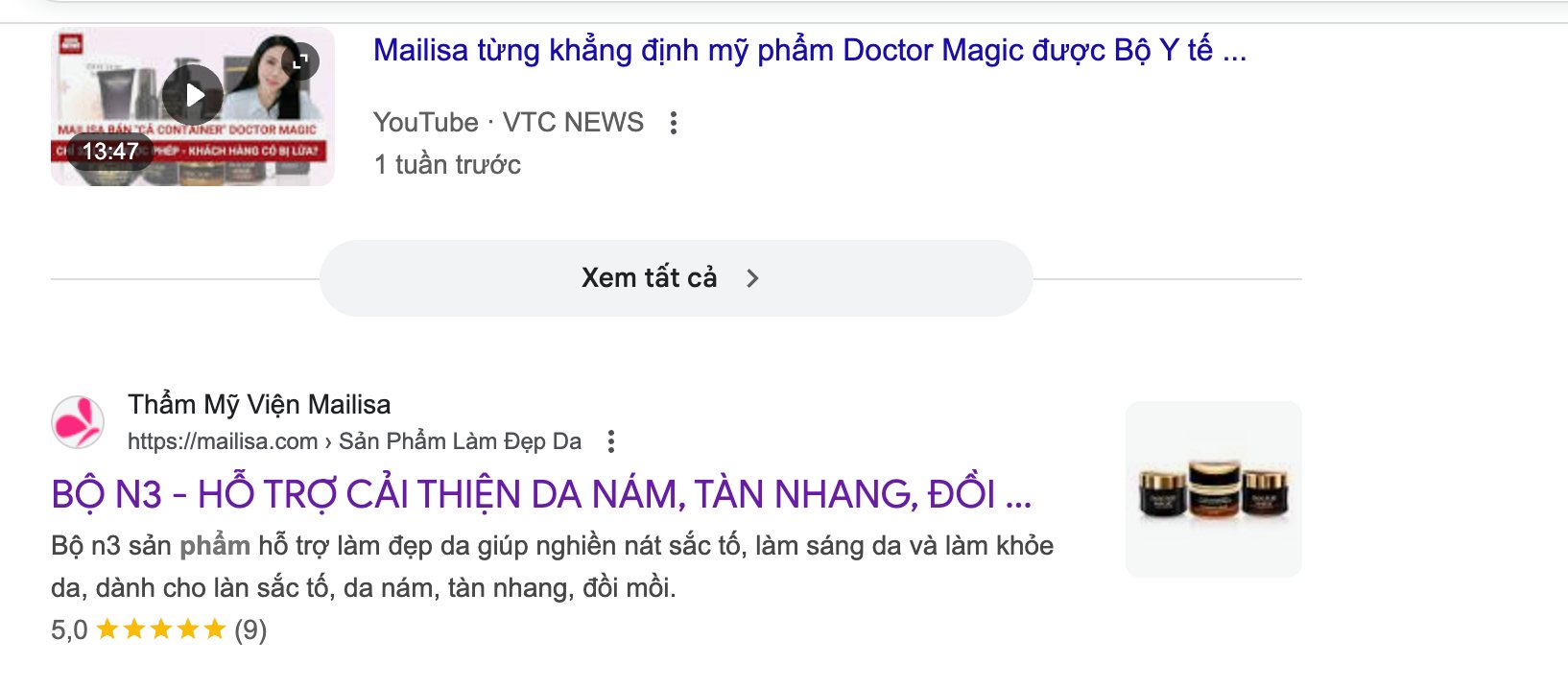
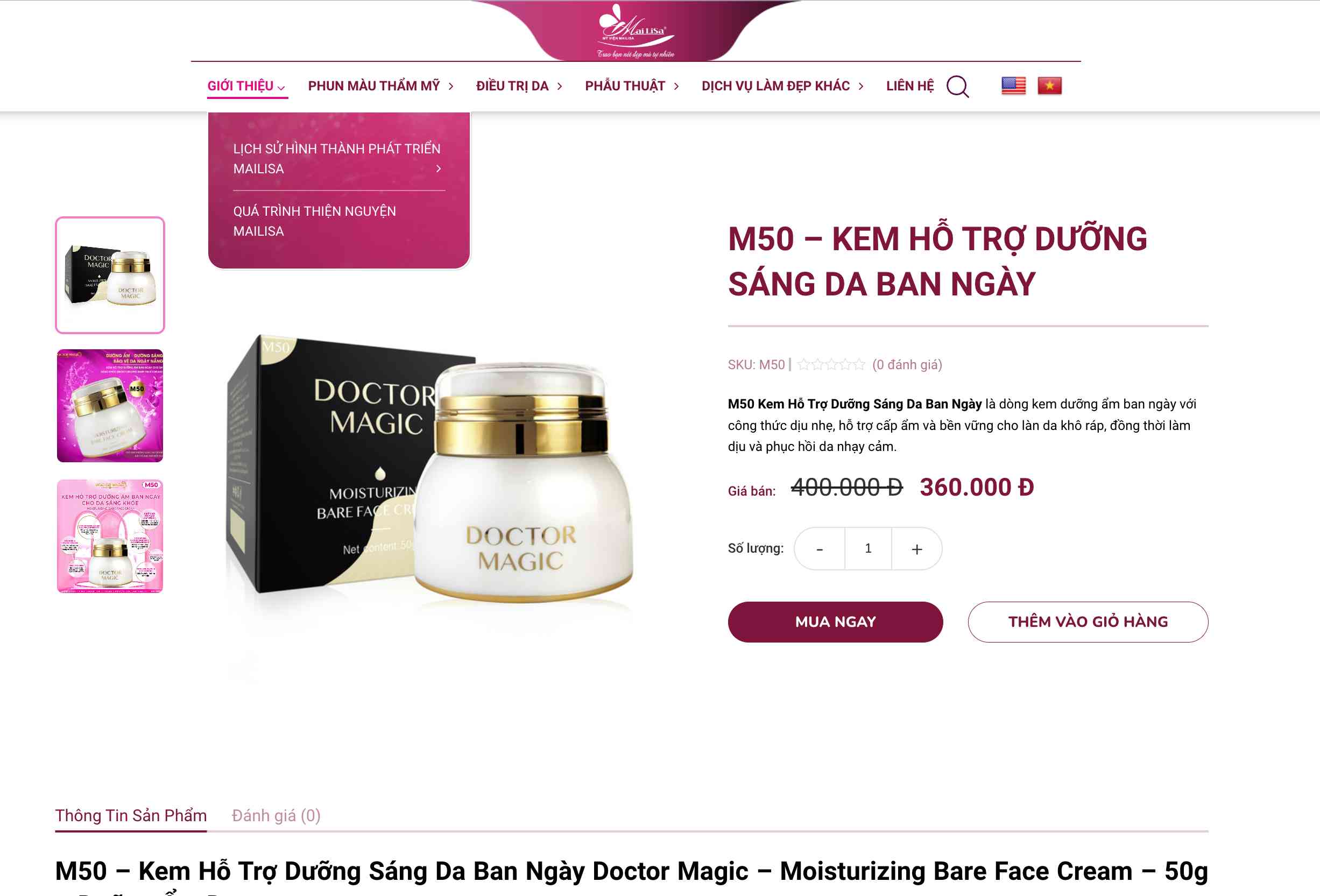
The main products of Mailisa beauty salon include the N3 set - a set of moisturizer to help improve melasma, velvet, and prey hill for sale at 2.3 million VND; Melasma Doctor Magic leather combo (N3+M23) for sale at 2.74 million VND; The B1 set - a set of moisturizer to help brighten the skin for 1.7 million VND still shows search results on Google.
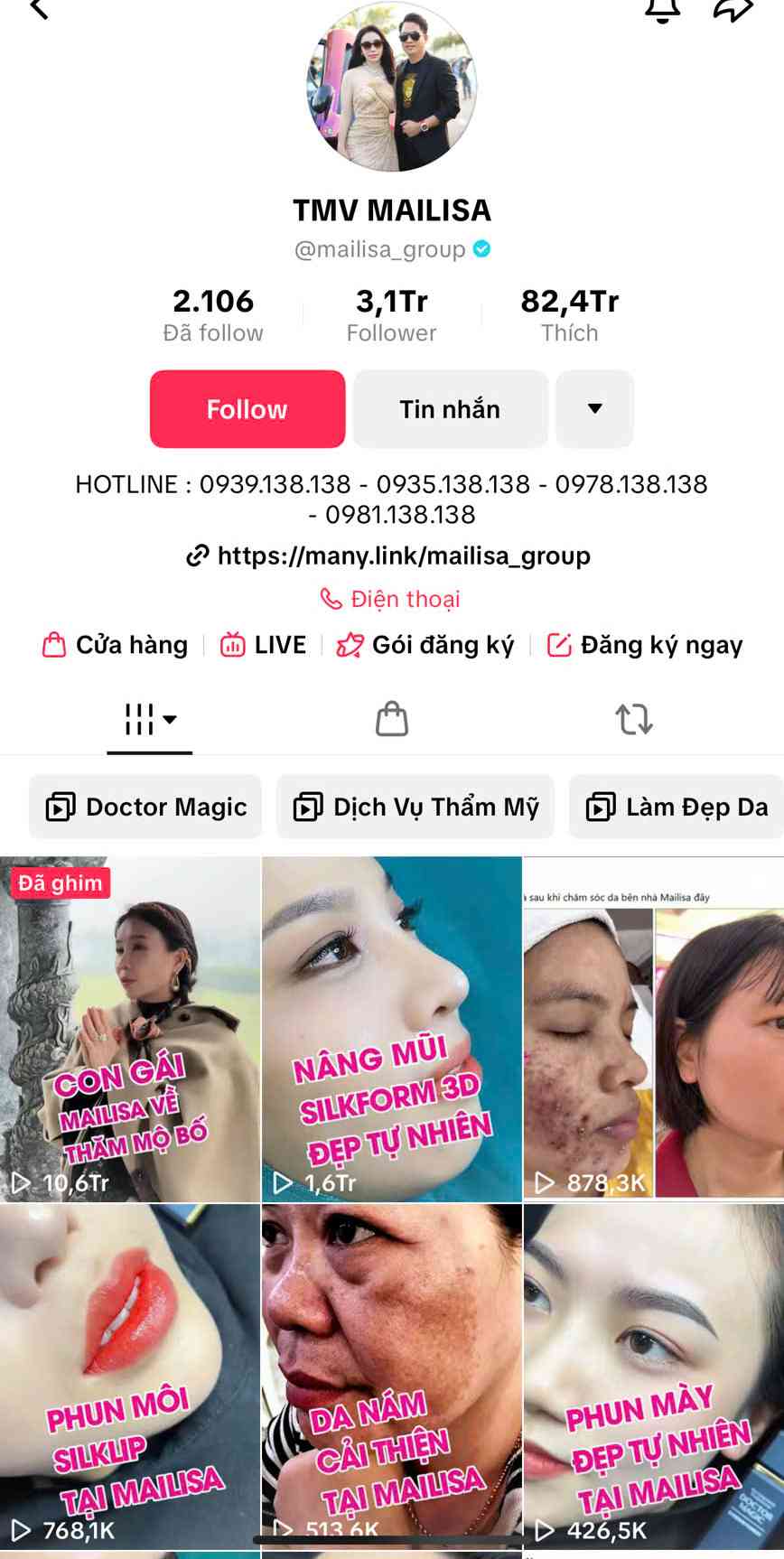
In addition, on the Facebook page THM M VIEN MAILISA with 2.8 million followers, as of November 21, the authorities prosecuted and arrested the accused to temporarily detain Phan Thi Mai, Director of Mailisa Cosmetic Company Limited, this page was still active and posted many advertisements. After November 22, this page has stopped updating.
Many of Mailisa's TikTok shopping baskets are empty, all products are hidden or removed even though the brand has previously livestreamed to sell Doctor Magic cosmetics and achieved huge sales. The MAILISA TMV Tiktok site with more than 3 million followers also stopped updating advertisements from November 22.
Dr. Ta Manh Hung, Deputy Director of the Department of Drug Administration (Ministry of Health) requested that cosmetic businesses and users nationwide immediately stop trading and using the above product lots and return them to product suppliers.
The Department of Health of provinces and cities notifies businesses and cosmetic users to recall the above cosmetic products, organize the reception of reports from production, business establishments, and users for recalled cosmetic products; preside over and coordinate with competent authorities to handle violating units in accordance with their authority and current regulations, and transfer the file to law enforcement agencies for handling in case of criminal violations.
According to Dr. Ta Manh Hung, when issuing the decision to revoke the receipt number of the declaration form and suspend the circulation of cosmetics of MK Skincare Import-Export Production and Trading Service Company Limited, the Department also sent a document requesting relevant units to coordinate in removing the product on e-commerce platforms.











