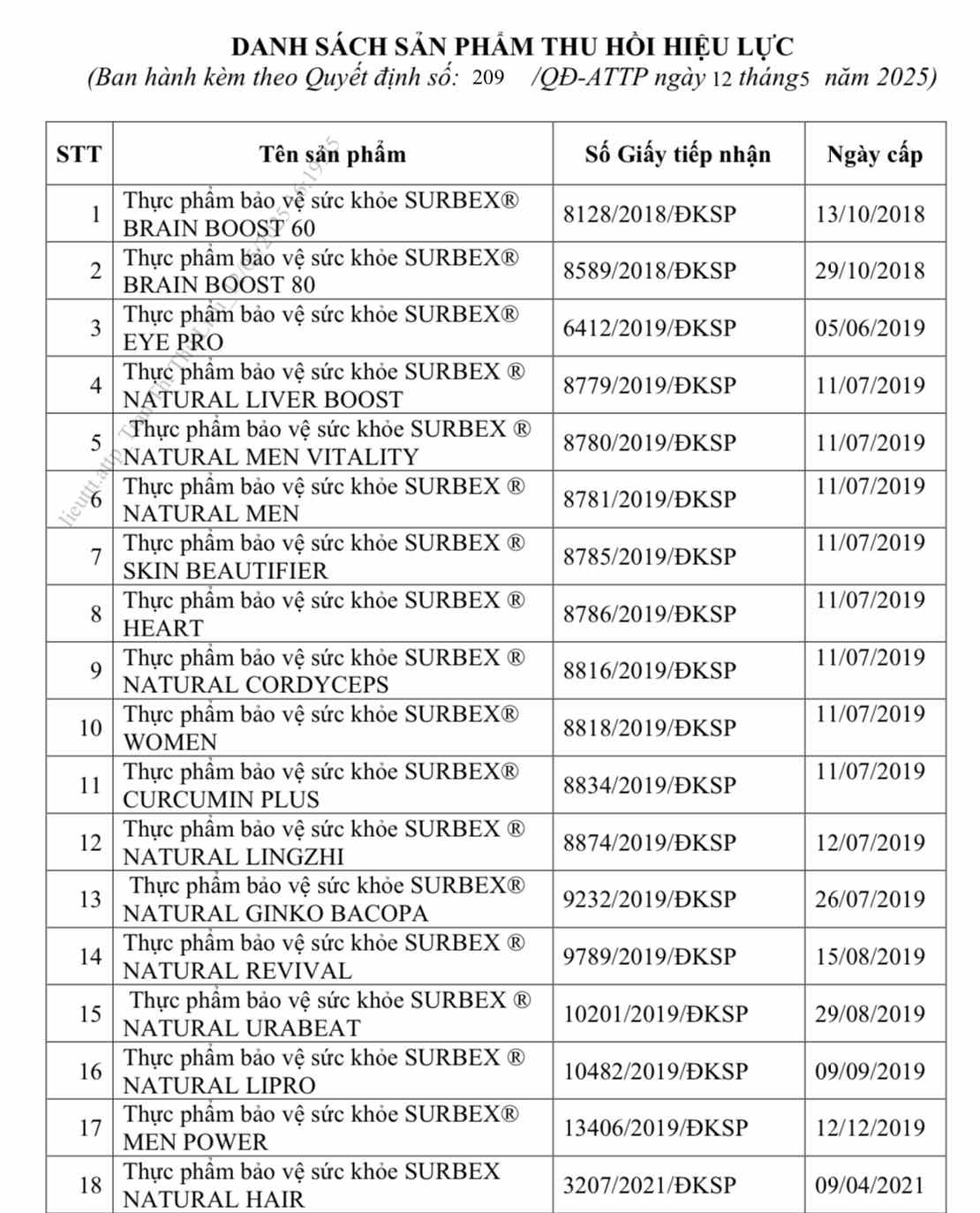
Dr. Chu Quoc Thinh, Deputy Director of the Department of Food Safety (Ministry of Health) has just signed a decision to revoke the validity of the Certificate of receipt of product declaration registration for 18 health protection food products of Abbott Healthcare Vietnam Company Limited.
These are products announced by the company - formerly known as Glomed Pharmaceutical Company Limited - located at 35 Thu Do Dai Lo, Vietnam - Singapore Industrial Park, An Phu Ward, Thuan An City, Binh Duong Province.
This decision takes effect from the date of signing and replaces Decision No. 187/QD-ATTP issued on April 29, 2025 on the same content of revocation to take effect.
The Food Safety Department requires Abbott Vietnam Company, the Food Product Management Department and relevant departments to be responsible for implementing the decision.
The products whose product declaration papers are revoked are in the list registered by this enterprise - formerly known as Glomed Pharmaceutical Company Limited.