According to SCMP, Revivicor, the biotechnology company led by Ayares, is at the forefront of xenotransplantation research - transplanting animal organs into humans. This research is aimed at solving the chronic organ shortage in the US.
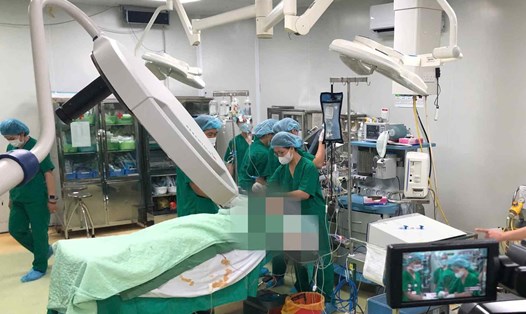
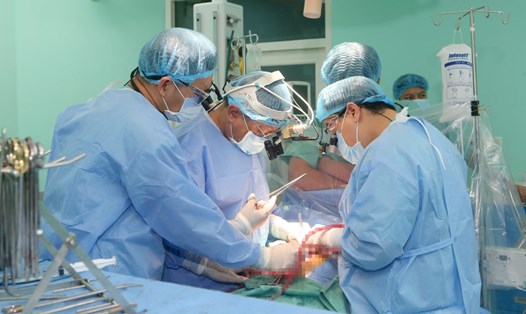
According to a December 17 announcement from a hospital in New York, Revivicor has successfully bred a pig with a kidney that can be transplanted to Towana Looney - a patient at the hospital.
Looney donated one kidney to his mother in 1999. However, just a few years later, her remaining kidney failed due to pregnancy complications.
The 53-year-old woman from Alabama is the latest patient to receive a gene-edited pig kidney, and the only person to survive after receiving an animal organ.
“It was a blessing,” she said three weeks after her surgery at NYU Langone.
For more than 20 years, Revivicor has been conducting pig-to-human transplant research in Blacksburg, Virginia. The pigs are genetically engineered so that their organs are less likely to be rejected by patients.
“These are not ordinary farm pigs. Millions of dollars have been invested in the production of these genetically modified pigs, so they are very high-value animals,” said Ayares.
While xenotransplantation research is ongoing in many parts of the world, the United States is one of the leaders in this field. The first strain of pigs developed by Revivicor carries only one gene that is modified to disable the production of a substance that causes humans to reject transplanted organs.
The second has 10 modified genes, six of which are derived from human DNA, to improve biocompatibility. And this type of pig is something that United Therapeutics (UT), Revivicor’s parent company, is planning to develop further.
Towana's case is a precursor to potential clinical trials, under Food and Drug Administration guidelines, to determine whether these organs are safe as a new source for human transplants and whether they are sustainable.
In March, the publicly traded company opened another medical facility near Blacksburg, where pigs’ kidneys will be removed and prepared for transfer to patients in the operating room. The rest of the pig will be discarded.
The company aims to begin clinical studies in patients in 2025, and if it gets the green light from the US Food and Drug Administration (FDA), it will begin large-scale production of the genetically modified pigs in 2029.
UT is now planning to invest billions of dollars to build more and larger facilities. According to Steadman, the company is considering selling kidneys for about $1 million each, which is roughly the cost of 10 years of dialysis for patients in the United States.