Concluding on the content of the denunciation against Mr. Le Van Dat, the Director of Can Tho City Dermatology Hospital pointed out the limitations of the Can Tho Department of Health in issuing Conclusion No. 818/KL-SYT in February 2025.
No procedures issued
According to the assessment and evaluation of the People's Committee of Can Tho City, the verification results show that previously, Can Tho City Dermatology Hospital (abbreviated as Hospital) treated skin diseases for patients using the needle rolling method. In 2019, the Hospital implemented the use of additional treatment methods using nutrient injection machines. However, due to ineffective implementation, the Hospital stopped using the nutrient injection machine.
In November 2022, Mr. Dat directed and assigned Ms. T.D.H to directly propose and be responsible for implementing the use of the treatment method using a nutrient injection machine.
Through inspection and review of the Hospital's professional technical list, there is no treatment for eczema and skin rejuvenation using nutrient injection machine technology. According to Mr. Dat's explanation, the procedure issued by the Hospital and approved by the Department of Health is the procedure for treating acne and skin aging with the needle rolling technique, not the procedure for using a nutrient injection machine. In fact, on September 27, 2024, the Hospital issued Decision No. 435/QD-BVDL on the Injection Technical Procedure - Department of Dermatology, including the process of using a nutrient injection machine.
The verification results, through working with the Head of the General Planning Department, confirmed that during the use and treatment of patients, the Hospital has not issued a procedure for using a nutrient injection machine. Therefore, the Hospital's treatment of phlegm and skin rejuvenation for patients using a nutrient injection machine from 2019 to the time of stopping use has not issued a procedure and has not been approved by the Department of Health for the technical list according to regulations.
Not yet approved by the professional manager
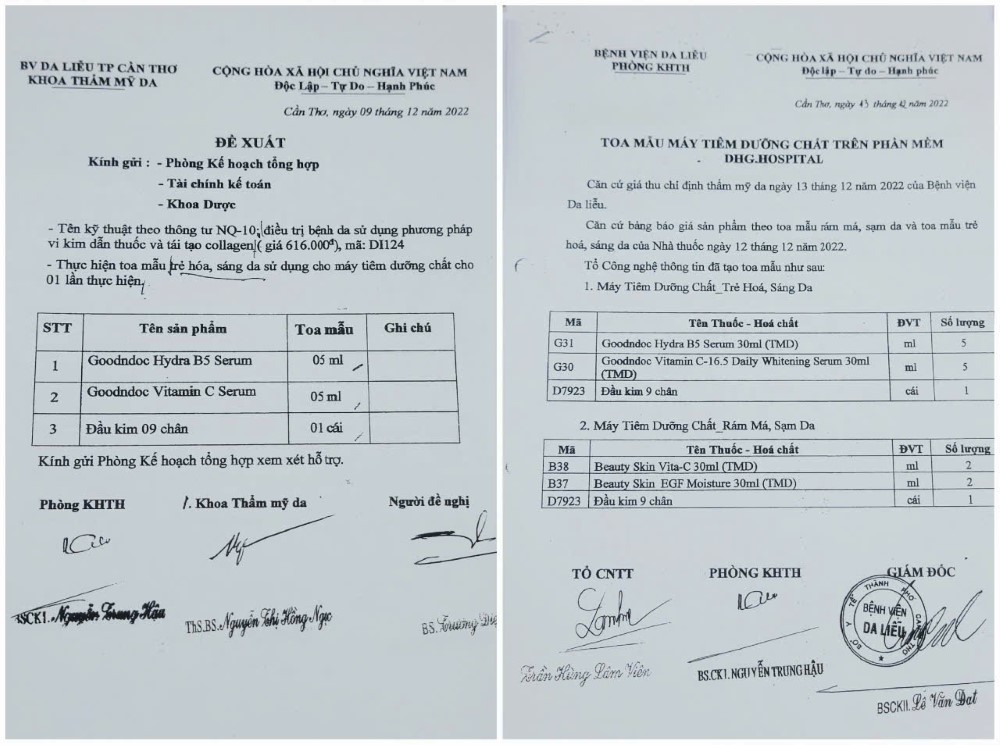
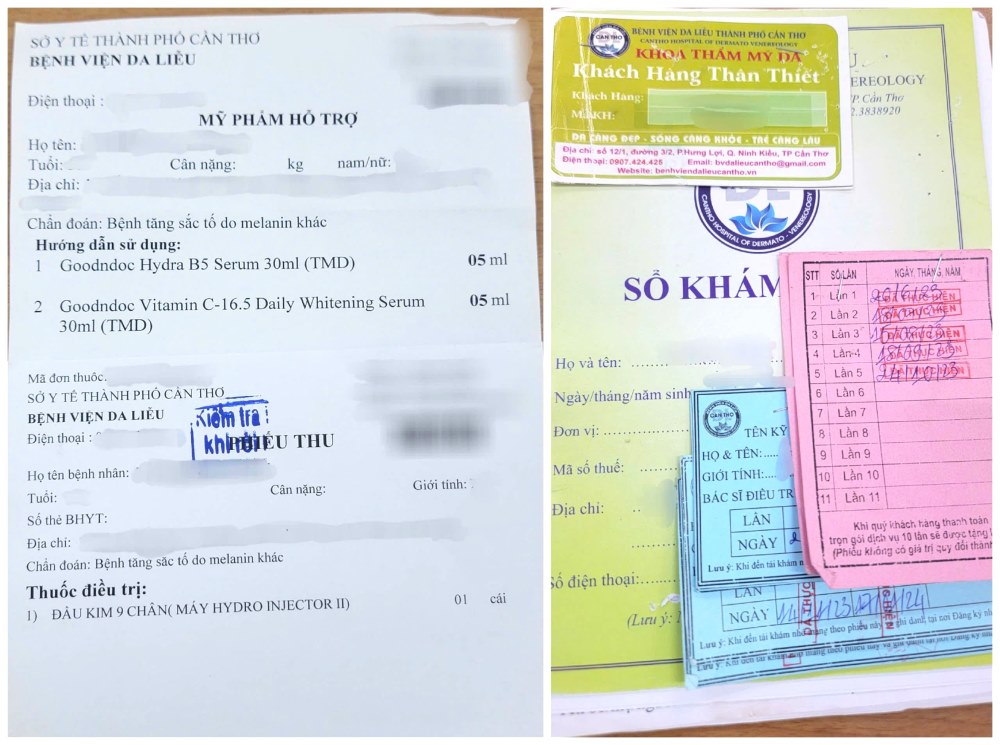
For the sample prescription of 4 cosmetic products used according to the proposal of Ms. T.D.H and the Department of Dermatology: Goodndoc Hydra B5 Serum, Goodndoc Vitamin C Serum, Beauty skin vita-C serum and Beauty skin EGF moisture, according to the cosmetic product declaration form, these 4 cosmetics are emulsion, milk, gel or oil used on the skin (face, hands, feet...).
The manufacturer's instructions for the 4 types of cosmetics do not have instructions or instructions for use as skin injections.
Through verification, the sample prescription has not been approved by the Science and Technology Council or the Hospital's Drug and Treatment Council and has not been approved by the Deputy Director in charge of expertise.
On the other hand, Mr. Dat and Ms. T.D.H explained that the proposal for a sample prescription was based on the needle formula table signed by the Deputy Director of the Hospital, but there was no document or evidence to prove it, so there was no basis for consideration.
A device used for injecting nutrients
Regarding the nutrient injection machine, through verification and working with a number of doctors assigned to treat patients using the nutrient injection machine, it shows that there is no consensus in determining the treatment method of directly injecting nutrients into the skin or applying them to the skin surface.
However, according to information, documents (Training Plan, information on Hydro Injector II) provided by Ms. T.D.H about the nutrient injection machine currently used at the Hospital show that this is a device used to inject nutrients through the skin, a different way of use and treatment compared to the needle rolling tool currently used at the Hospital.
Mr. Dat and Ms. T.D.H explained that this is a treatment using a mechanism to increase skin permeability, not that the skin injection method has no basis to prove it.
Thus, Ms. T.D.H is the person assigned to be directly responsible for implementing the treatment method using a nutrient injection machine. Ms. H proposed a sample prescription of 4 types of cosmetics but did not follow the manufacturer's instructions.

For the Hospital Director, knowing the information that the product was a skin cosmetic, he signed a prescription for the sample. When there was information, Mr. Dat did not direct the inspection, clarification or organization of a meeting of the Professional Council to assess whether the use of skin-injected cosmetics was right or wrong to take appropriate treatment measures. Mr. Dat also reported to the Department of Health and explained that Ms. T.D.H's sample prescription proposal was correct while not fully directing the implementation of professional and technical measures.
Also according to the conclusion of the People's Committee of Can Tho City, Mr. Dat showed signs of covering up Ms. T.D.H in the proposal to use cosmetics to inject into the skin with a nutrient machine. The accusation is well-founded and the accusation is correct. The main responsibility belongs to the Hospital Director, Deputy Head of the Dermatology Department in charge of management.
In November 2024, Lao Dong Newspaper published a series of articles on Can Tho City Dermatology Hospital prescribing 4 types of topical cosmetics to inject into patients' faces from the treatment method of a nutrient injection machine. The cosmetic import and distribution company confirmed to reporters that the product could only be applied to the skin by hand, while at that time, Specialist Doctor 2 Le Van Dat - Director of Can Tho City Dermatology Hospital - said that the way to "increase permeability" was correct and 100% safe.
On the patient's side, about 10 months of treatment (including the use of other drugs) cost about 46 million VND, but the skin condition did not improve significantly. In fact, with this treatment, patients have to pay more than buying the above cosmetics to apply to the skin.