The Department of Drug Administration (Ministry of Health) has just issued a decision to suspend the circulation and recall nationwide of 20 cosmetic products of Dai Cat A International Company Limited, address: 43D/44 Ho Van Hue, Ward 9, Phu Nhuan District, Ho Chi Minh City.
According to the Drug Administration, through inspection on May 28, 2025, it was discovered that 2 products including Queendoes Rhea Serum and Renewing concentrate had formulas that were not as advertised in the records. In addition, 18 other products have labels that are not in accordance with the disclosure documents.
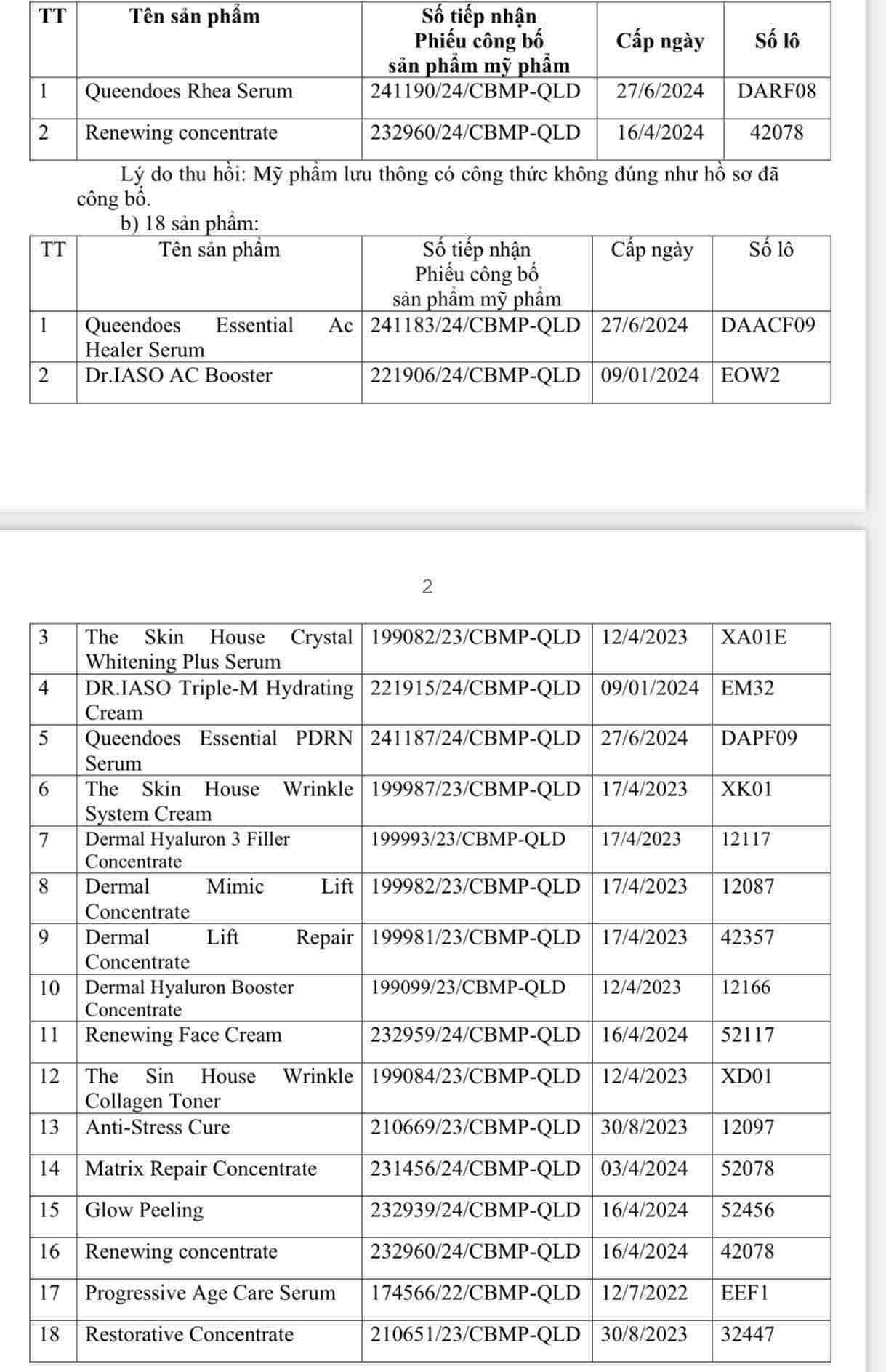
To ensure consumer rights and strictly manage the production and trading of cosmetics, the Drug Administration of Vietnam requires the Departments of Health of provinces and cities to notify businesses and users to immediately stop circulating the above products and organize the recall of all violating products.
Dai Cat A International Company Limited is responsible for sending a revocation notice to the distributors, receiving the returned product and destroying it according to regulations if the violating factor cannot be eliminated. The report on the results of the recall must be sent to the Department of Drug Administration before June 30, 2025.
The Ho Chi Minh City Department of Health is assigned to supervise the entire recall process and report to the Drug Administration before July 10, 2025.











