On February 28, the Can Tho Department of Health issued a conclusion after verification of the previous incident of skin-on-the- skin vaccination reported by Lao Dong Newspaper.
According to the conclusion, the act violated the Law on Medical Examination and Treatment No. 15/2023/QH15. The responsibility belongs to Dr. T.D.H, leader of the Department of Dermatology, leader of the Financial Planning Department and the hospital's Board of Directors.
"The violation of the Law on Medical Examination, Treatment and Use of Cosmetics without the manufacturer's instructions was announced to be systematic from the treating physician to the departments and the Board of Directors over many years. The management of the hospital's professional activities is not in-depth, lacking inspection, leading to professional violations that negatively affect the image and expertise of the hospital even though there have been no complications for patients" - the conclusion clearly stated.

The conclusion of the Can Tho Department of Health also shows that at Can Tho Dermatology Hospital, there has been a situation of trading and using 12 cosmetic products that have not been granted a cosmetic product declaration form by the Department of Drug Administration of the Ministry of Health. This responsibility belongs to the team of experts in bidding, the pharmacist in charge of the pharmacy, the pharmacy management board and the hospital director himself.
The conclusion of the Can Tho Department of Health also includes the contents of taking advantage of position and power; revoking documents in violation of regulations; signing labor contracts that cause collective impacts... for the Director of Can Tho Dermatology Hospital.
Regarding the handling measures that need to be implemented and recommended by the competent authority, the Can Tho Department of Health will review and organize a review of the responsibilities of individuals, collectives and the Board of Directors of Can Tho Dermatology Hospital according to its authority for the shortcomings and limitations in the case of directing the import of invalid paper cosmetics (suspected forgery) into the hospital for use, violating financial management principles and other contents.
Reviewing and handling responsibilities for individuals, groups and the Board of Directors of Can Tho Dermatology Hospital according to their authority for the shortcomings, limiting the use of cosmetic injections on the skin.
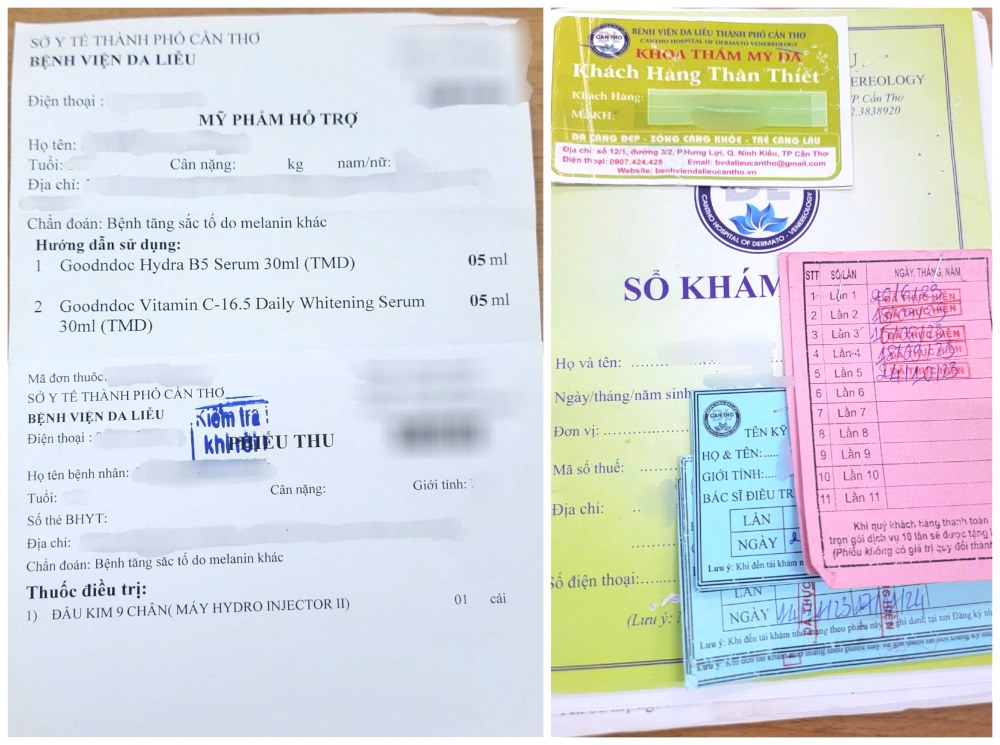
As Lao Dong reported in the article Suspected hospital in Can Tho injecting cosmetics into the skin, according to the patient's reflection, Can Tho Dermatology Hospital used 4 types of cosmetics to apply to the sample prescription, using a Hydro Injector II nutrient injection machine to inject into the patient's face for a long time.
According to the reporter's investigation, in the cosmetic product declaration form issued by the Department of Drug Administration (Ministry of Health) and instructions for use, all 4 products above are in the form of "ker, emulsion, milk, gel or oil used on the skin (face, feet, hands, etc.).
Specialist Doctor 2 Le Van Dat - Director of Can Tho Dermatology Hospital - informed Lao Dong: "The Ministry of Health has granted a license to circulate the product and we also use the product according to the manufacturer's instructions. The manufacturer allows application to put nutrients deep into the skin, when the manufacturer says against the indication to put them in the skin, this method cannot be used. We affirm that the product and technique are 100% safe, if not suitable, red skin or acne is not recommended."
Meanwhile, the representative of the cosmetics import and distribution company affirmed that it is not allowed to apply nutrients to the skin by hand, even very small encroachments.











