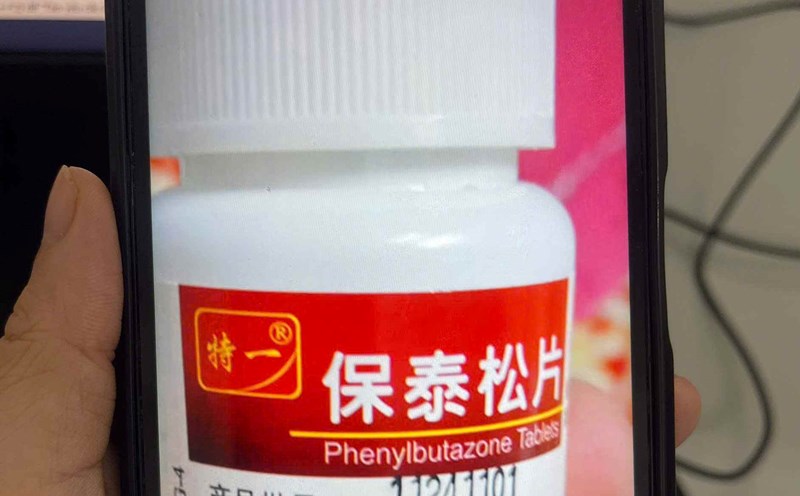
According to the announcement from Bach Mai Hospital, in the past time, the hospital has received and treated many cases of severe allergies related to the arbitrary use of products containing the active ingredient Phenylbutazone advertised for sale on social networks. Many patients have dangerous complications such as high fever, rash, bleeding, acute liver failure, and have even had deaths. These products are of unknown origin, not licensed for circulation, but are still advertised as joint pain medicine.
Bach Mai Hospital said that allergy cases often start late, at least after a week, or even last for 1 - 3 months from the time of taking the drug. This makes it difficult to exploit the history of the disease and determine the cause. In addition, the product name is often in foreign languages, making it difficult for patients to remember or read accurately, delaying the diagnosis and timely intervention process. Initial symptoms are also easily confused with infectious diseases, causing patients to often go to other specialties for examination, leading to prolonged diagnosis and not being treated early.
According to data from the Drug Administration of Vietnam (Ministry of Health), currently in Vietnam, there are no drugs containing the active ingredient Phenylbutazone that are granted a certificate of circulation registration, and there is no license to import raw materials or finished products containing this active ingredient.
To prevent the risk, the Drug Administration has requested the provincial and municipal Departments of Health to urgently notify all businesses and people to absolutely not buy, sell or use drugs containing Phenylbutazone or any products of unknown origin. When detecting suspicious products, organizations and individuals must immediately report to the authorities for inspection and handling.
Strengthen propaganda so that people do not arbitrarily buy medicine through social networks or by word of mouth, but must check and buy medicine at legal facilities.
Closely coordinate with the police, market management, and Steering Committee 389 to investigate and strictly handle acts of drug trafficking of unknown origin, especially false advertising in the online environment.
Request pharmaceutical businesses and hospitals to review and remove false advertisements about Phenylbutazone, while complying with legal regulations on drug origin.