Recently, the Department of Drug Administration, Ministry of Health has decided to withdraw 11 cosmetic product declaration receipt numbers of LUPACELL International Pharmaceutical and Cosmetic Joint Stock Company.
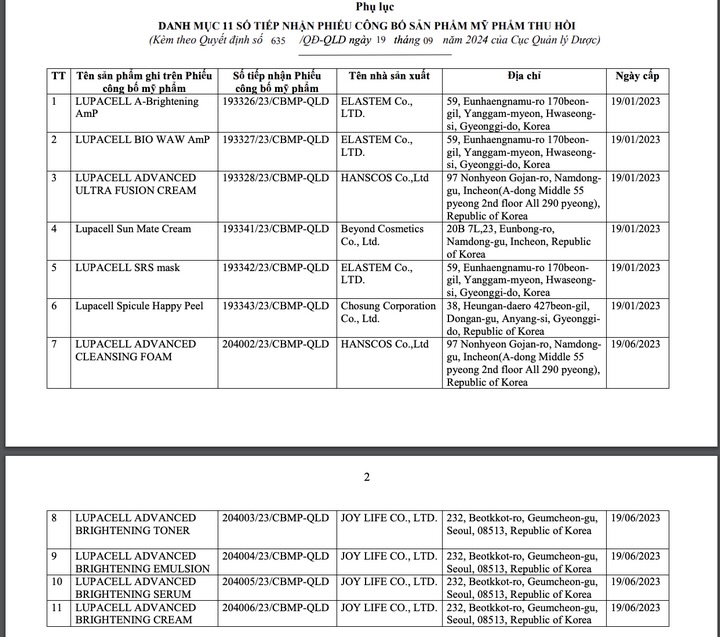
Cosmetic products whose declarations have been recalled include: Lupacell A-Brightening AmP (declaration number 193326/23/CBMP-QLD January 19, 2023); Lupacell Bio Waw AmP (declaration number 193327/23/CBMP-QLD January 19, 2023); Lupacell Advanced Ultra Fusion Cream (declaration number 193328/23/CBMP-QLD January 19, 2023);
Lupacell Sun Mate Cream (announcement number 193341/23/CBMP-QLD January 19, 2023); Lupacell S.R.S Mask (announcement number 193342/23/CBMP-QLD January 19, 2023); Lupacell Spicule Happy Peel (announcement number 193343/23/CBMP-QLD January 19, 2023; Lupacell Advanced cleansing Foam (announcement number 204002/23/CBMP-QLD June 19, 2023)... and other products.
According to the Drug Administration, the declaration forms of beauty and hair care products brought to market by LUPACELL International Pharmaceutical and Cosmetic Joint Stock Company were revoked for the reason of dishonest declaration of the contents in the cosmetic product declaration forms.
It is known that according to the regulations of the Ministry of Health, each cosmetic product when put into circulation on the market must have a product information file (PIF - Product Information File) according to ASEAN guidelines kept at the address of the organization or individual responsible for putting the product on the market.
Cosmetic product information file includes: Administrative documents and product summary; Quality of raw materials; Quality of finished products; Safety and effectiveness.
According to regulations, the administrative documents and product summary of this Dossier must be presented immediately to the inspection and examination agency upon request; other parts, if incomplete, must be presented within 15-60 days from the date of inspection upon request of the competent authority.
The Cosmetic Product Declaration Receipt Number is the number issued by the competent state management agency upon receipt of the cosmetic product declaration dossier. This number is valid to certify that the cosmetic product has been declared by the organization or individual responsible for bringing the product to the market to the competent state management agency regarding the cosmetic product being circulated on the market. It is not valid to certify that the product ensures safety, effectiveness, and meets all requirements of the ASEAN Cosmetic Agreement and its Annexes.










