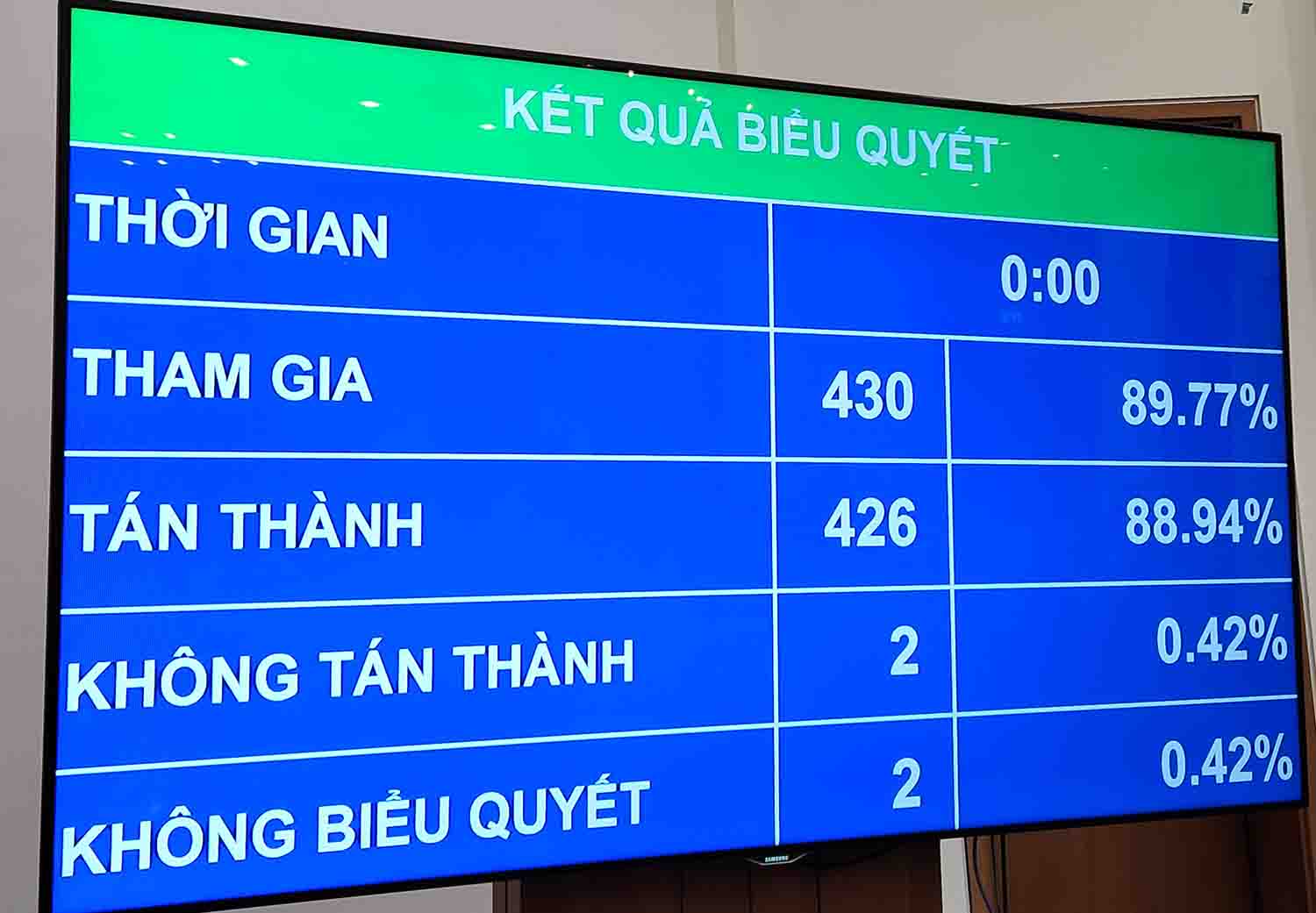
On November 21, continuing the 8th Session, the National Assembly voted to pass the Law amending and supplementing a number of articles of the Law on Pharmacy. The majority of delegates voted to pass at this Session.
Of which, 426/430 delegates voted in favor; 2 delegates disagreed and 2 delegates did not vote.
The law will take effect from 1.7.2025, however some provisions will take effect from 1.1.2025.
The new Pharmacy Law passed by the National Assembly has regulations on pharmaceutical trading via e-commerce.
According to the law, trading in drugs through e-commerce is prohibited for prescription drugs (except in cases of medical isolation for infectious diseases in group A); drugs subject to special control and drugs on the list of drugs restricted from retail sale.
Establishments trading in drugs and pharmaceutical ingredients using e-commerce methods must sell on e-commerce trading floors, e-commerce applications, and websites with online ordering functions.
Units must comply with the law on advertising and consumer protection; keep buyer information confidential.
Units are also required to post certificates of eligibility for pharmaceutical business, pharmaceutical practice certificates, and information about approved drugs.
In addition, drug retail establishments must provide online consultation and instructions on how to use the drugs to buyers and deliver the drugs to buyers according to detailed instructions of the Minister of Health.
Previously, on behalf of the National Assembly Standing Committee, reporting on the acceptance of explanations, Chairwoman of the Social Committee Nguyen Thuy Anh said that there were opinions suggesting specific regulations on the responsibilities of e-commerce trading floors in controlling the quality and origin of drugs sold on the floors.
The Standing Committee of the National Assembly finds that the responsibilities of e-commerce trading floors have been stipulated in the law on e-commerce and e-transactions.
In addition, this is only a means to conduct transactions, pharmaceutical business activities will still be carried out by pharmaceutical business establishments.
Therefore, the establishments selling drugs on the platform must be responsible for the quality of the drugs, similar to the traditional form of buying and selling. The law on e-commerce and electronic transactions also stipulates the responsibilities of the parties on the e-commerce trading platform.
Regulations such as the draft law are also consistent with the management trend of e-commerce in the pharmaceutical business in a number of countries around the world.











