Recently, the Ministry of Health issued an official dispatch to temporarily suspend the review and acceptance of cosmetic product declaration dossiers for KEIN Import-Export Joint Stock Company (address: 63 Van Phuc, Van Phuc Ward, Ha Dong District, Hanoi) for 6 months.
The reason given by the Drug Administration of Vietnam is that KEIN Import-Export Joint Stock Company did not have a product information file (PIF) stored at the enterprise as prescribed, violated point l, clause 1, Article 47 of Circular No. 06/2011/TT-BYT and dishonestly declared the contents in the cosmetic product declaration form, violating point k, clause 1, Article 47 of Circular No. 06/2011/TT-BYT dated January 25, 2011 of the Ministry of Health regulating the management of cosmetics.
This is not the only move by the Ministry of Health to strictly handle violations by this company.
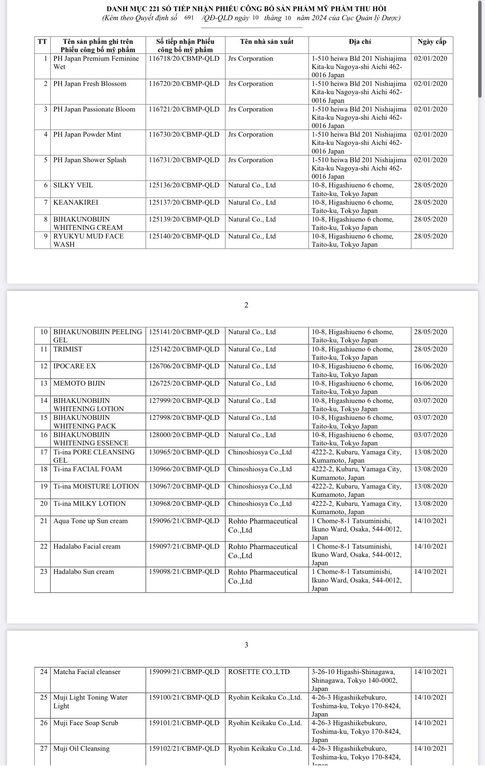
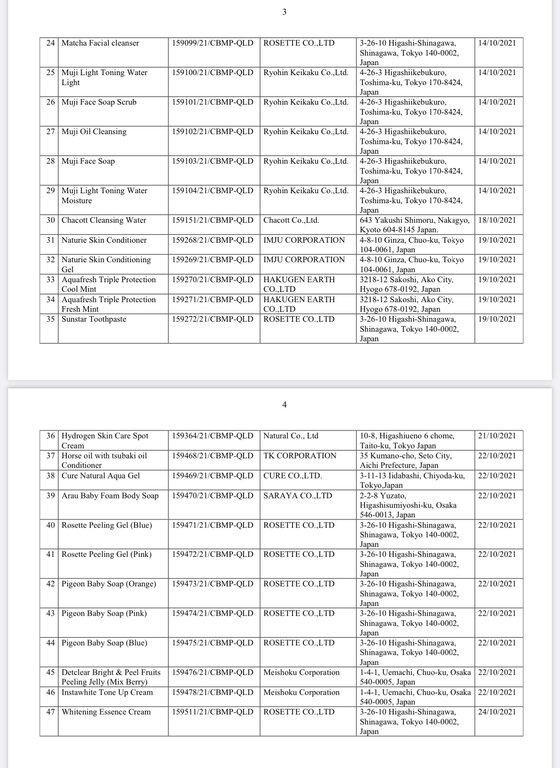
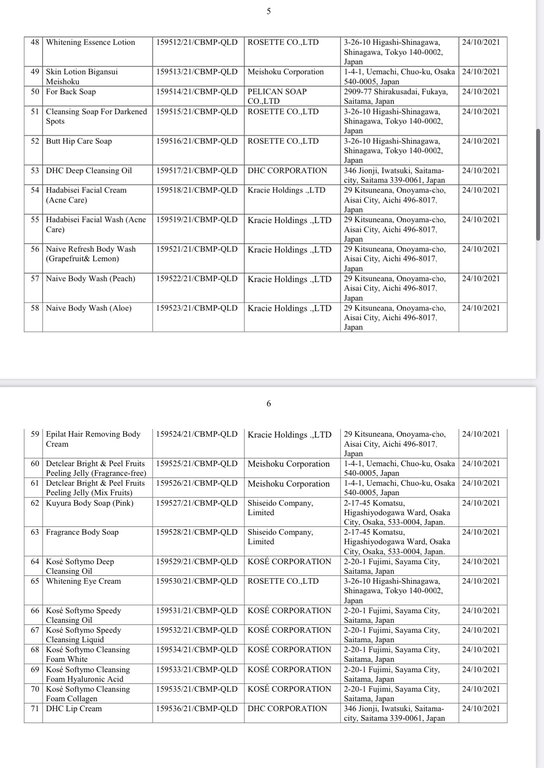
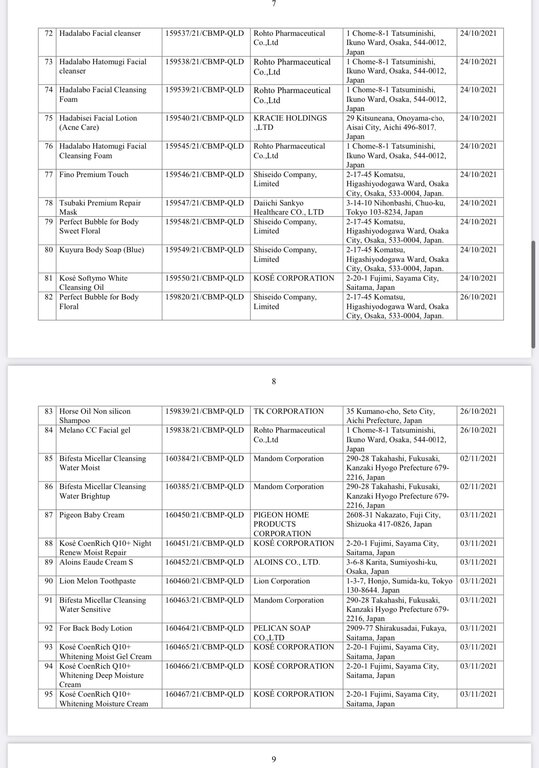
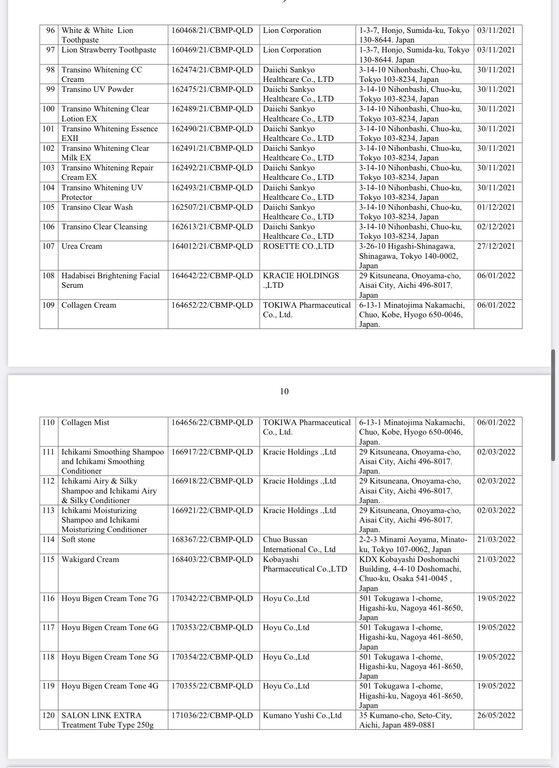
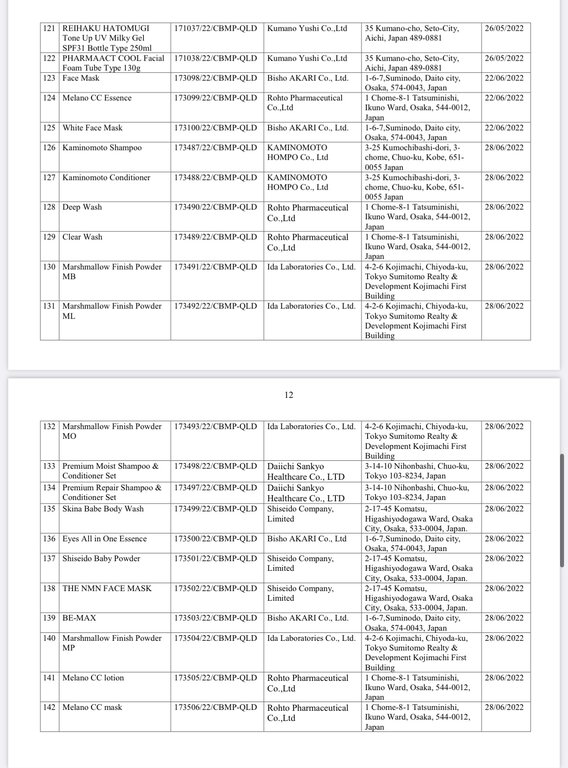
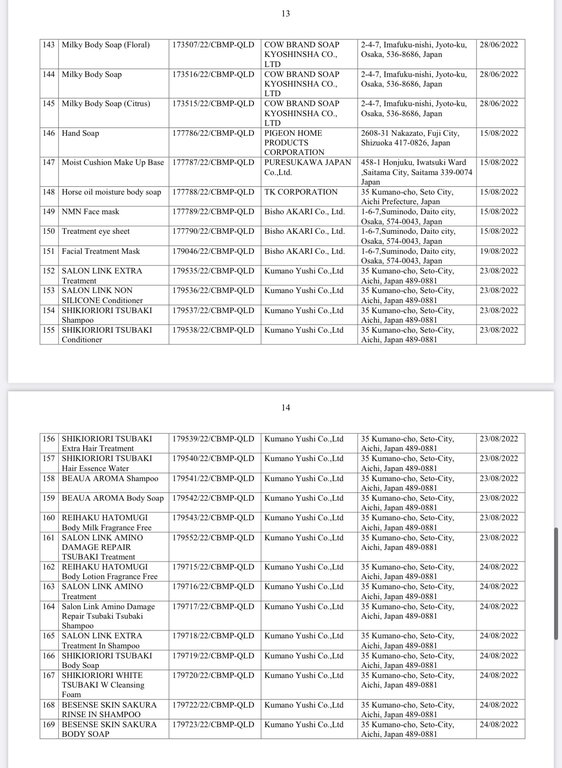
Regarding the Cosmetic Product Declaration Form, previously, the Drug Administration of Vietnam also issued Decision No. 691-QD/QLD on the revocation of 221 Cosmetic Product Declaration Forms received from KEIN Import-Export Joint Stock Company due to "no product information records presented to the competent authority as prescribed, dishonest declaration of the contents in the Cosmetic Product Declaration Form".
According to the reporter's investigation, the 221 products recalled by the KEIN Import-Export Joint Stock Company are all skin care products manufactured in Japan.
Below are details of 221 cosmetic products whose declaration receipt numbers were revoked by this enterprise.
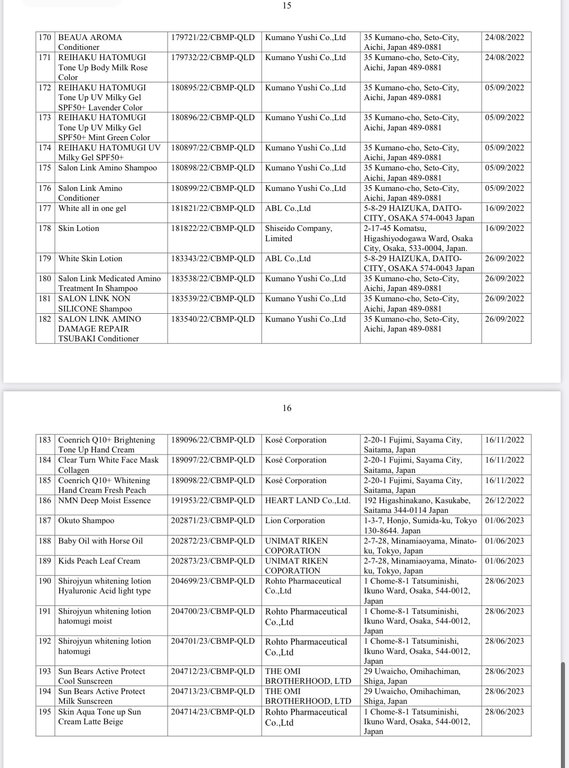
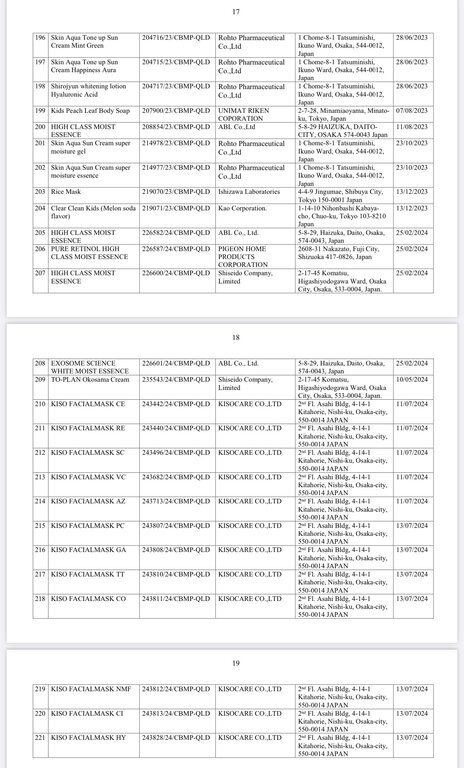
The competent state management agency will temporarily suspend the review and acceptance of cosmetic product declaration dossiers for 6 months for organizations and individuals committing one of the following acts:
- Trading in smuggled cosmetics, fake cosmetics, cosmetics of unknown origin;
- Trading in cosmetics that have not been granted a Cosmetic Product Declaration receipt number by a competent state management agency;
- Failure to recall violating cosmetics as notified by competent state agencies;
- Producing and trading cosmetics at establishments that do not comply with the principles and standards of "Good Manufacturing Practice for Cosmetics" of the Association of Southeast Asian Nations (CGMP-ASEAN) or equivalent recognized by the ASEAN Cosmetic Council;
- Manufacturing and trading cosmetics containing banned substances used in cosmetics or exceeding the permitted limits for substances with prescribed concentrations and usage limits according to current regulations of law;
- Using raw materials to produce cosmetics that have been banned from circulation on the market by the manufacturing country;
- Importing and trading cosmetics or cosmetic production materials that have been banned from circulation on the market by the manufacturing country;