On November 18, the Department of Drug Administration (Ministry of Health) issued a document requesting MK Skincare Import-Export Production and Trading Service Company Limited, address K31, Thoi An Residential Area, Le Thi Rieng Street, Thoi An Ward, Ho Chi Minh City - the enterprise owned by Mr. Hoang Kim Khanh, Ms. Mailisa's husband - to provide information on test results and product information records.
To serve the work of the competent authority for cosmetic products organized by MK Skincare Import-Export Production and Trading Service Company Limited and responsible for bringing them to the market under the brands "DOCTOR MAGIC", "MAIKA BEAUTY", "MK", the Drug Administration requested the Company to provide a report on the testing; stating that the agencies/units have tested the related products; sending the test results of the products; report on the product information file (PIF).
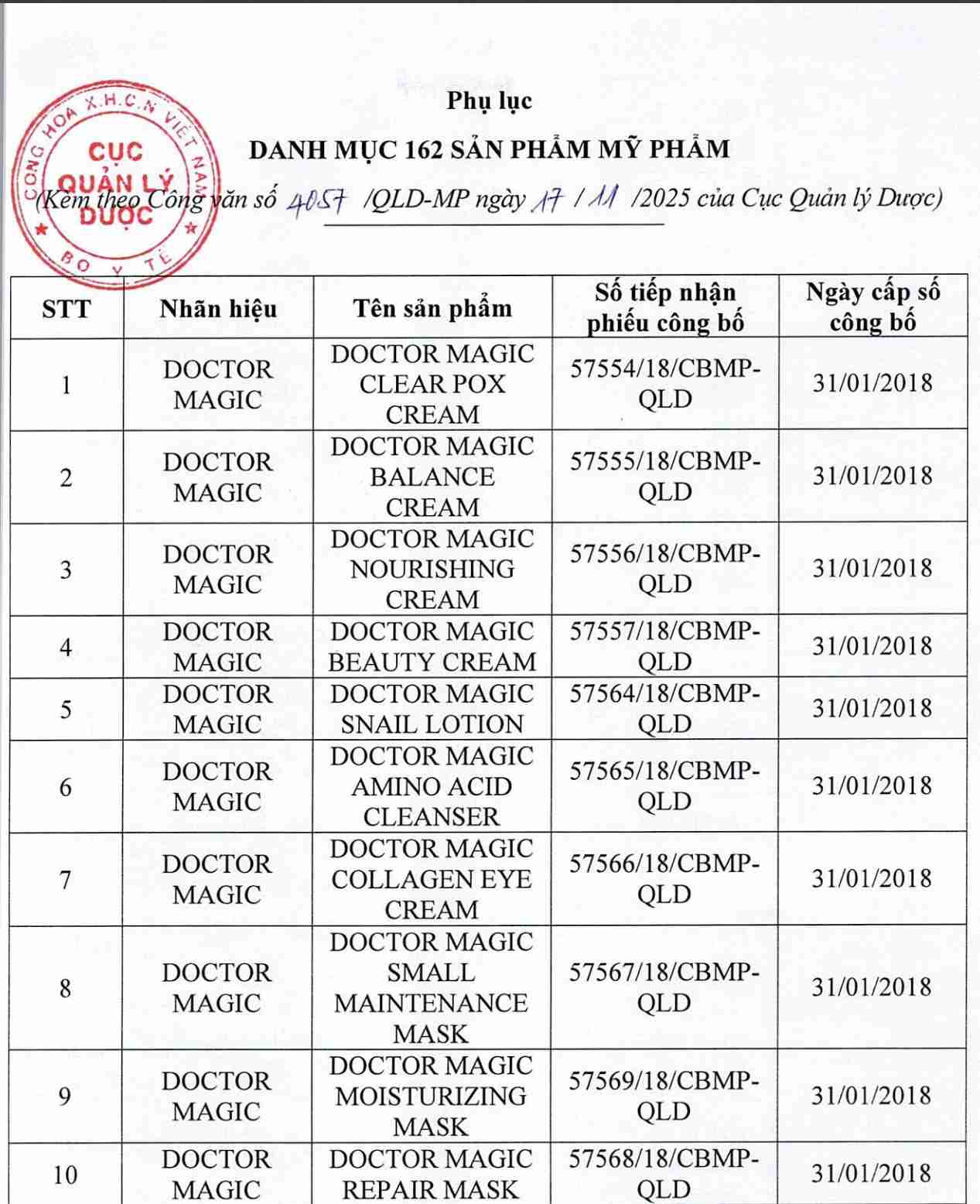
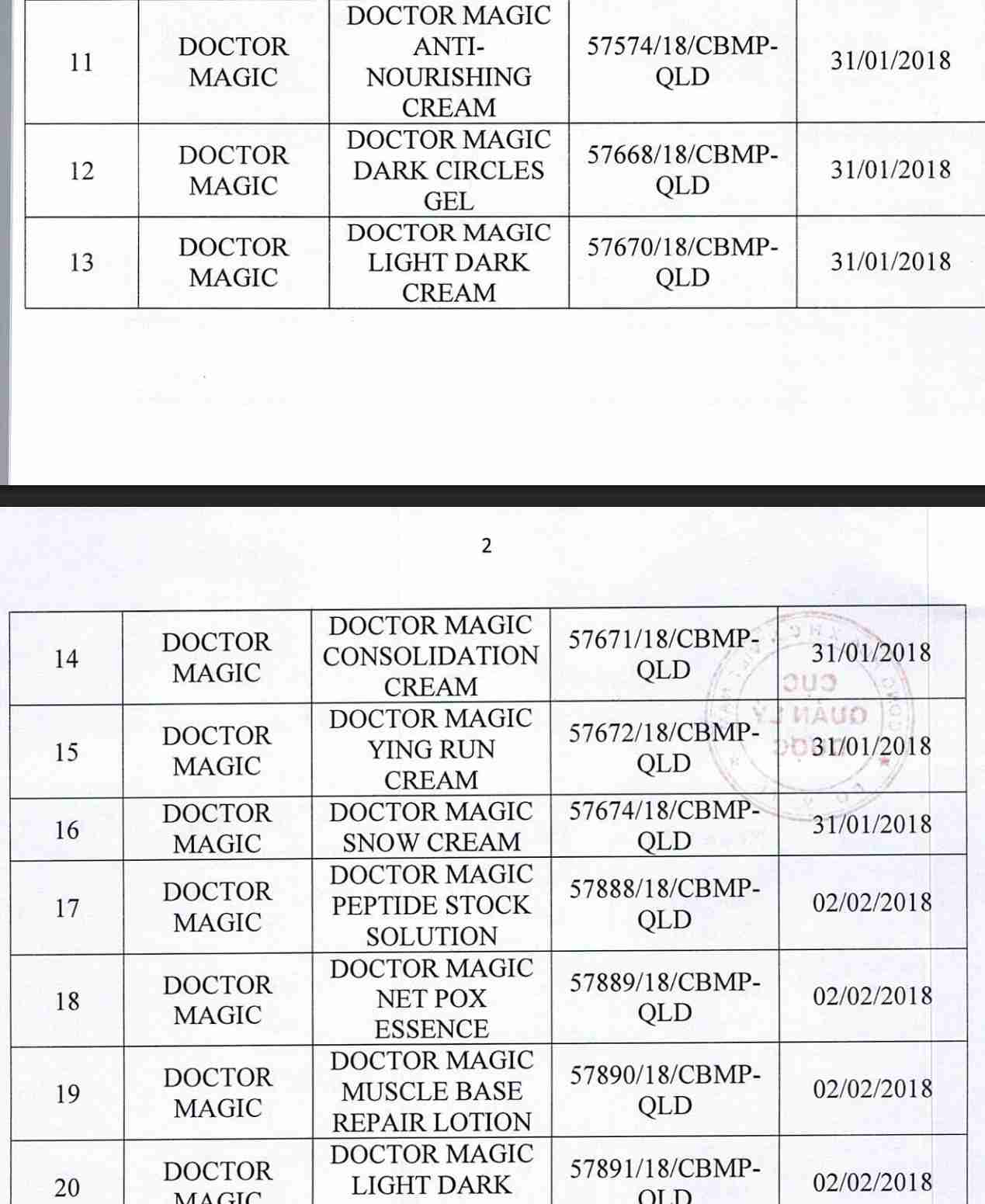
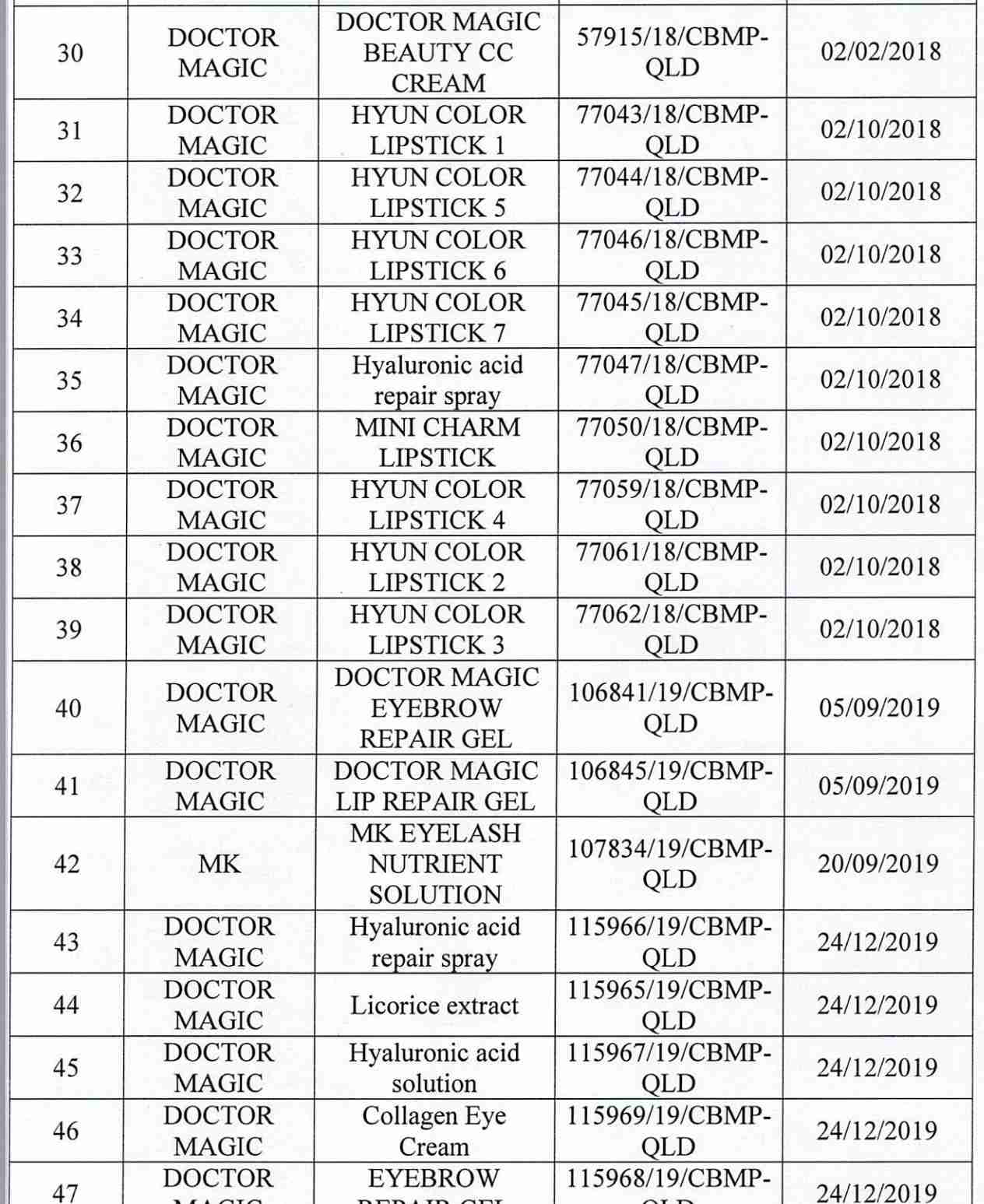
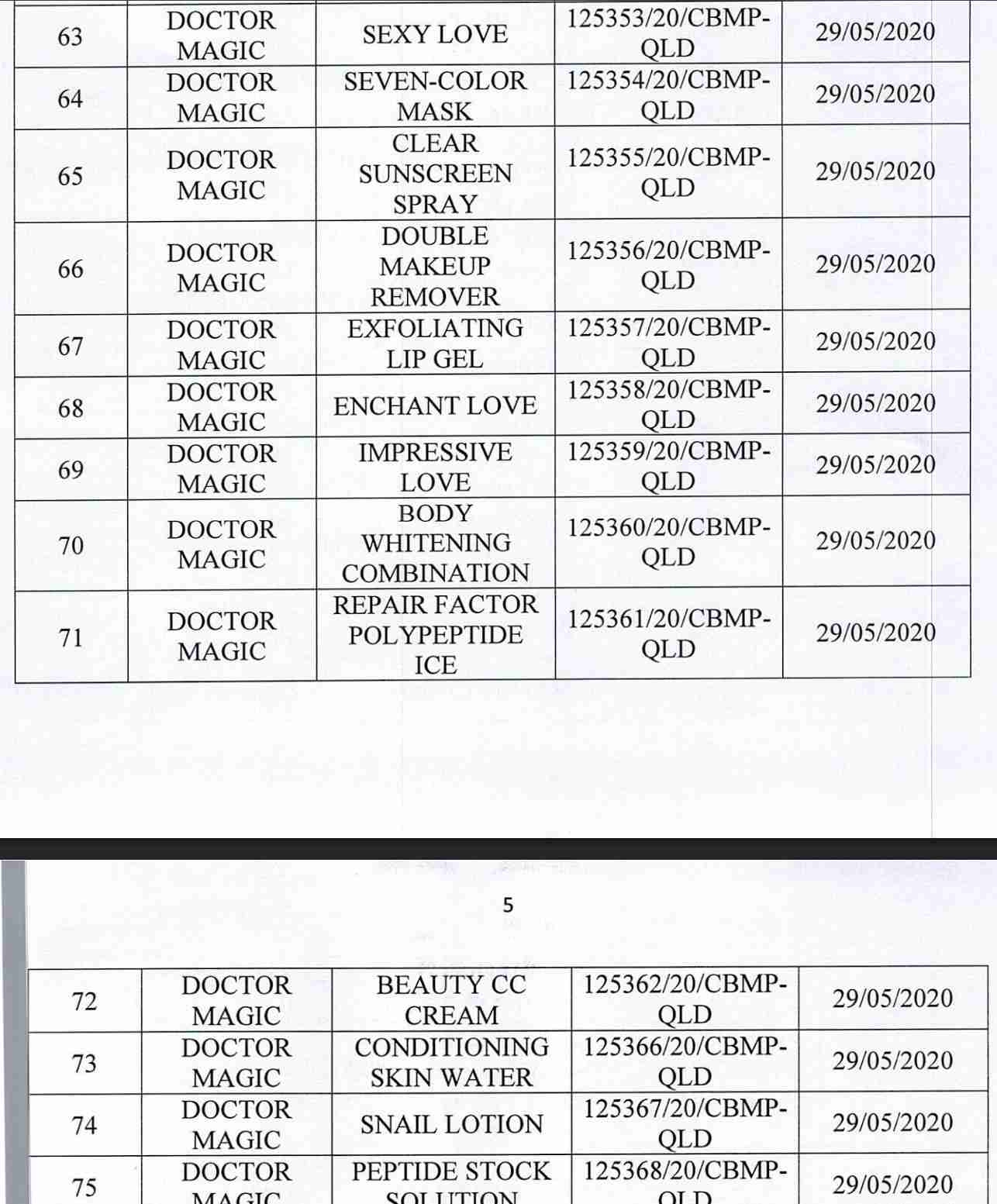
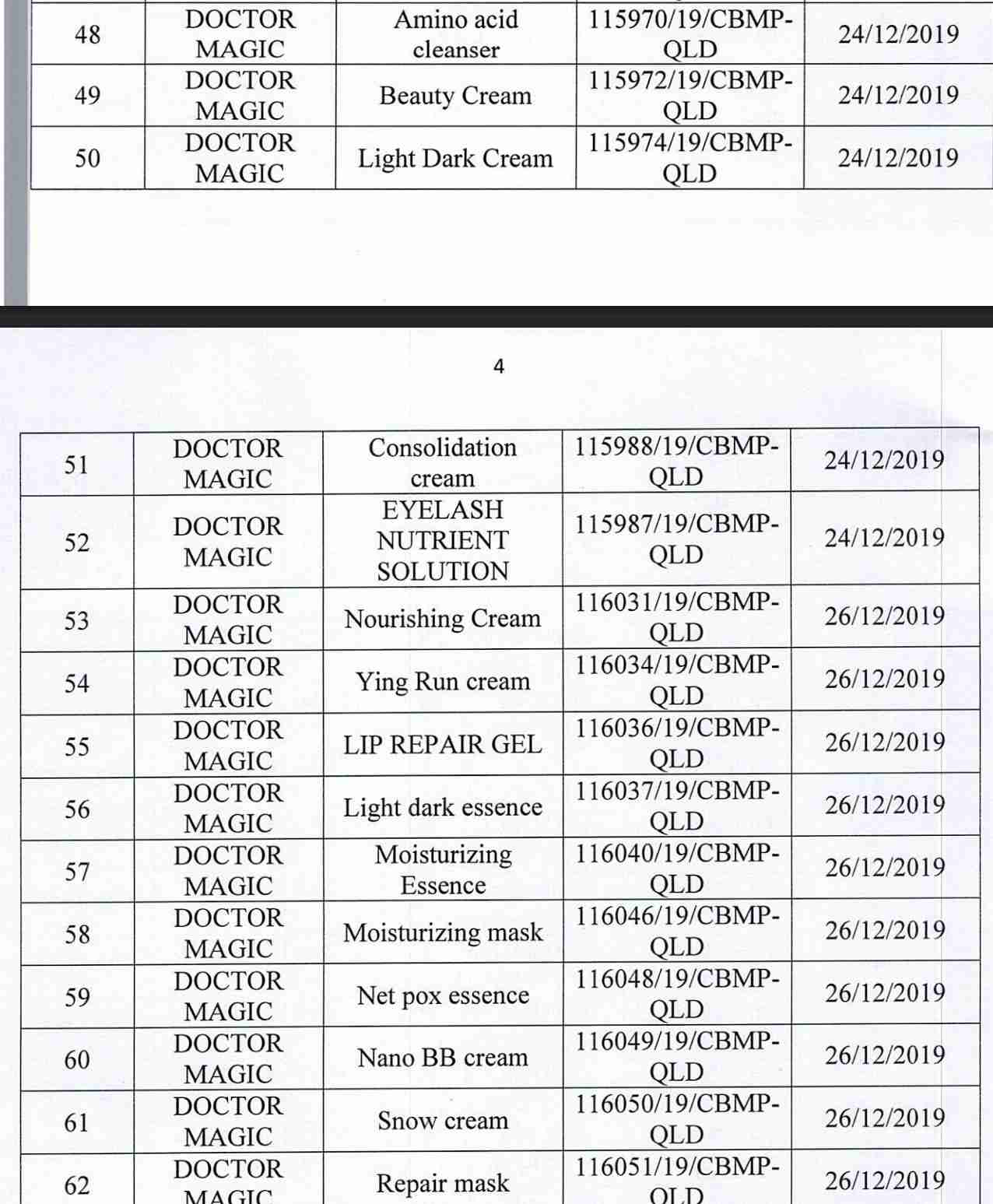
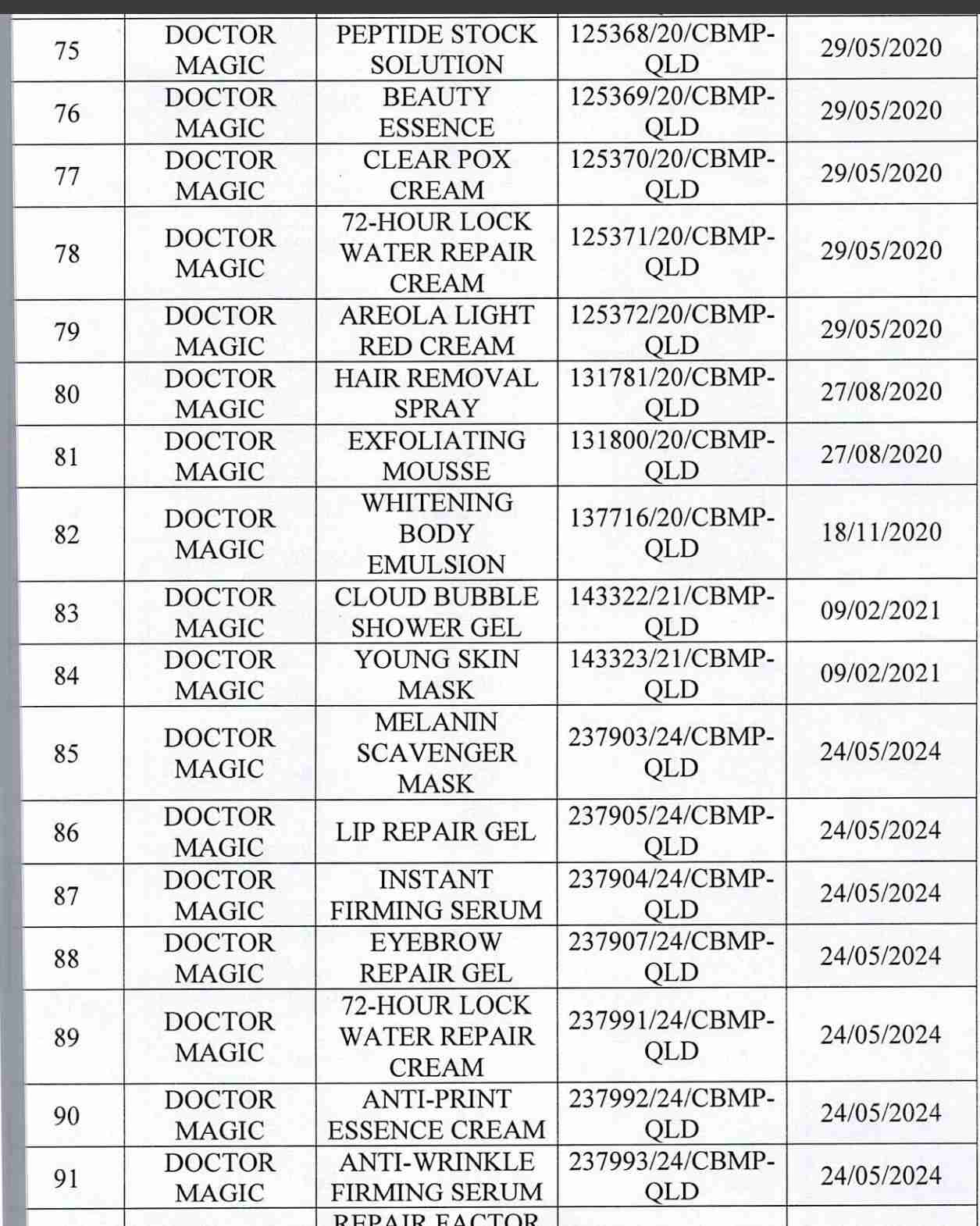
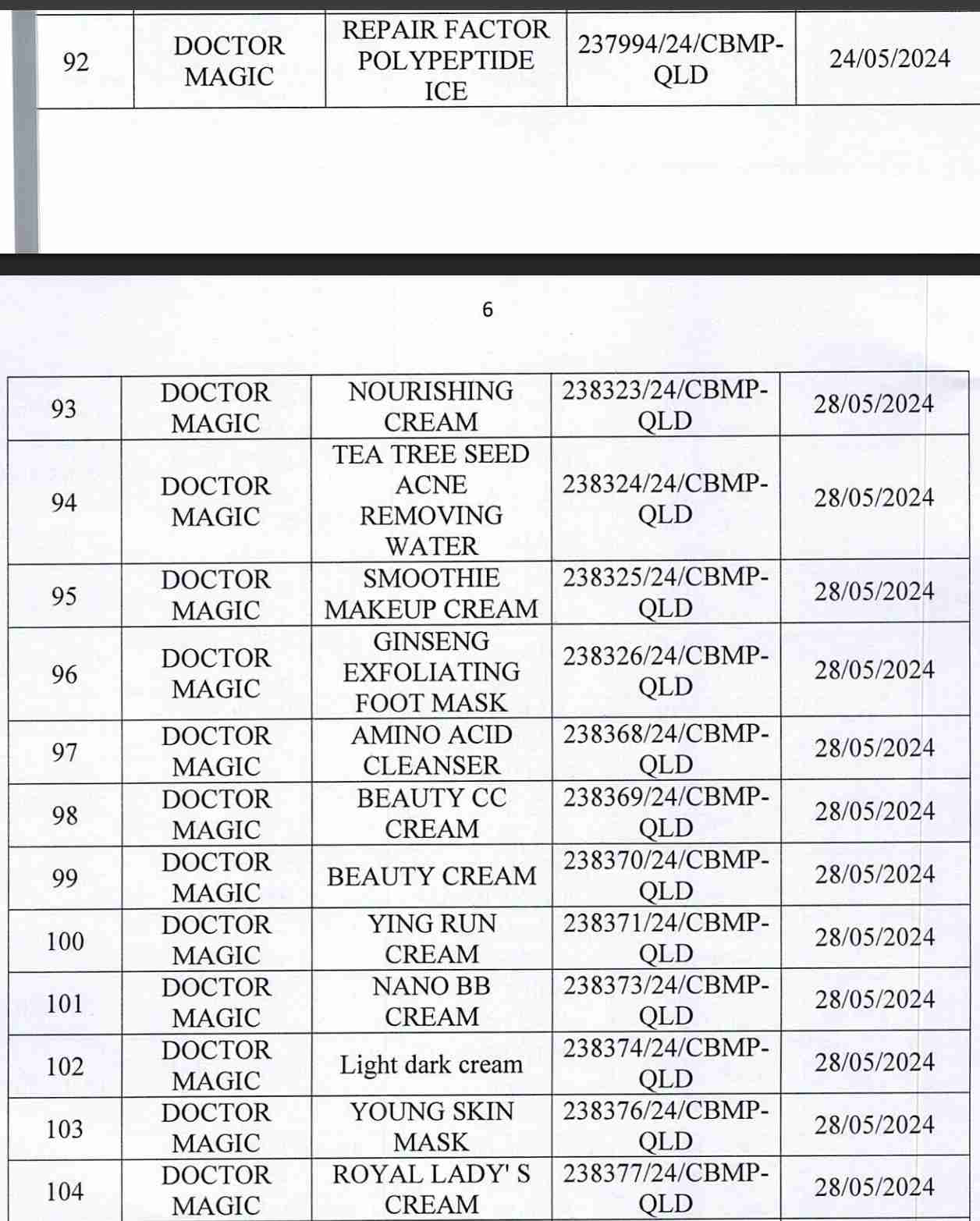
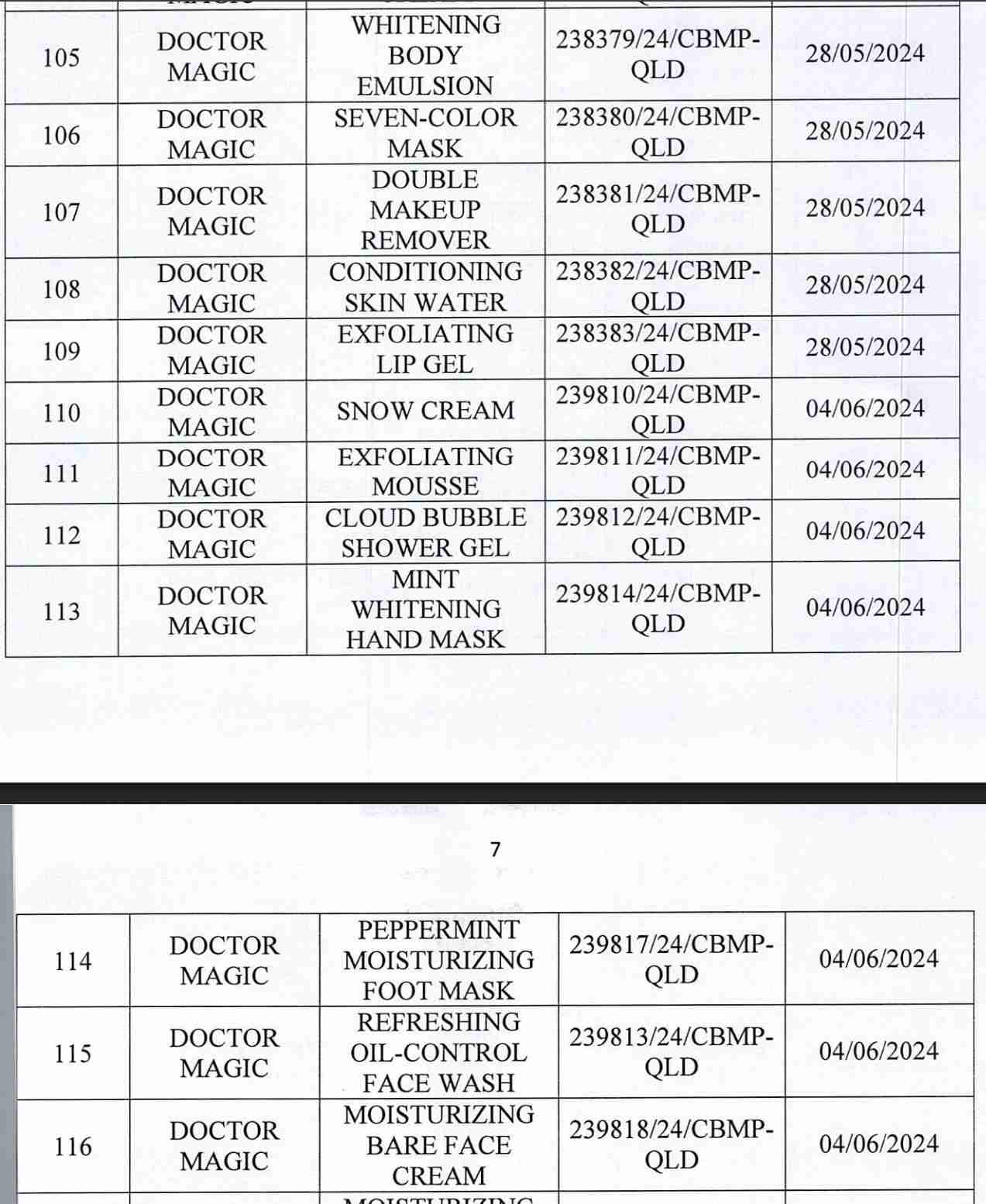
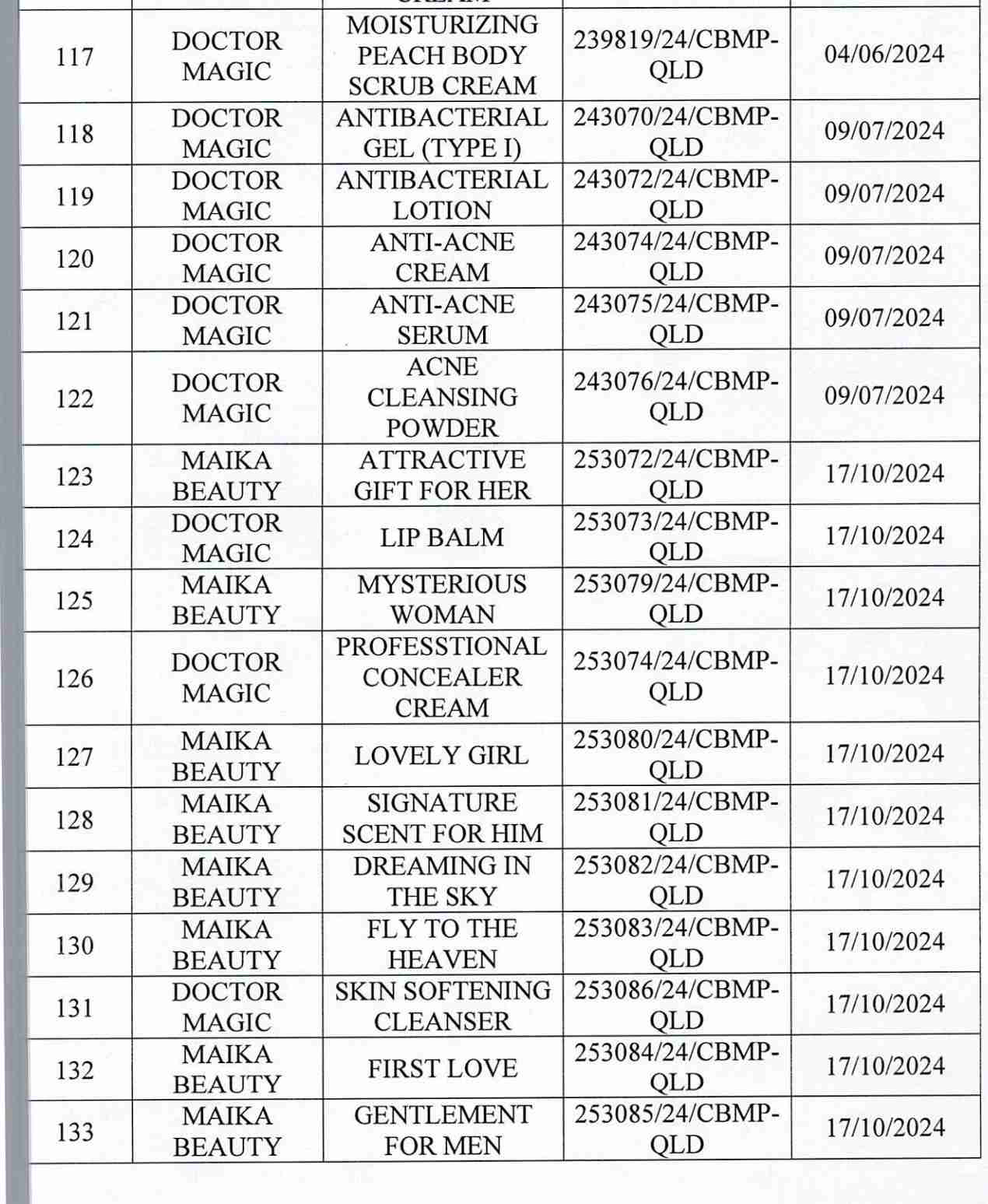
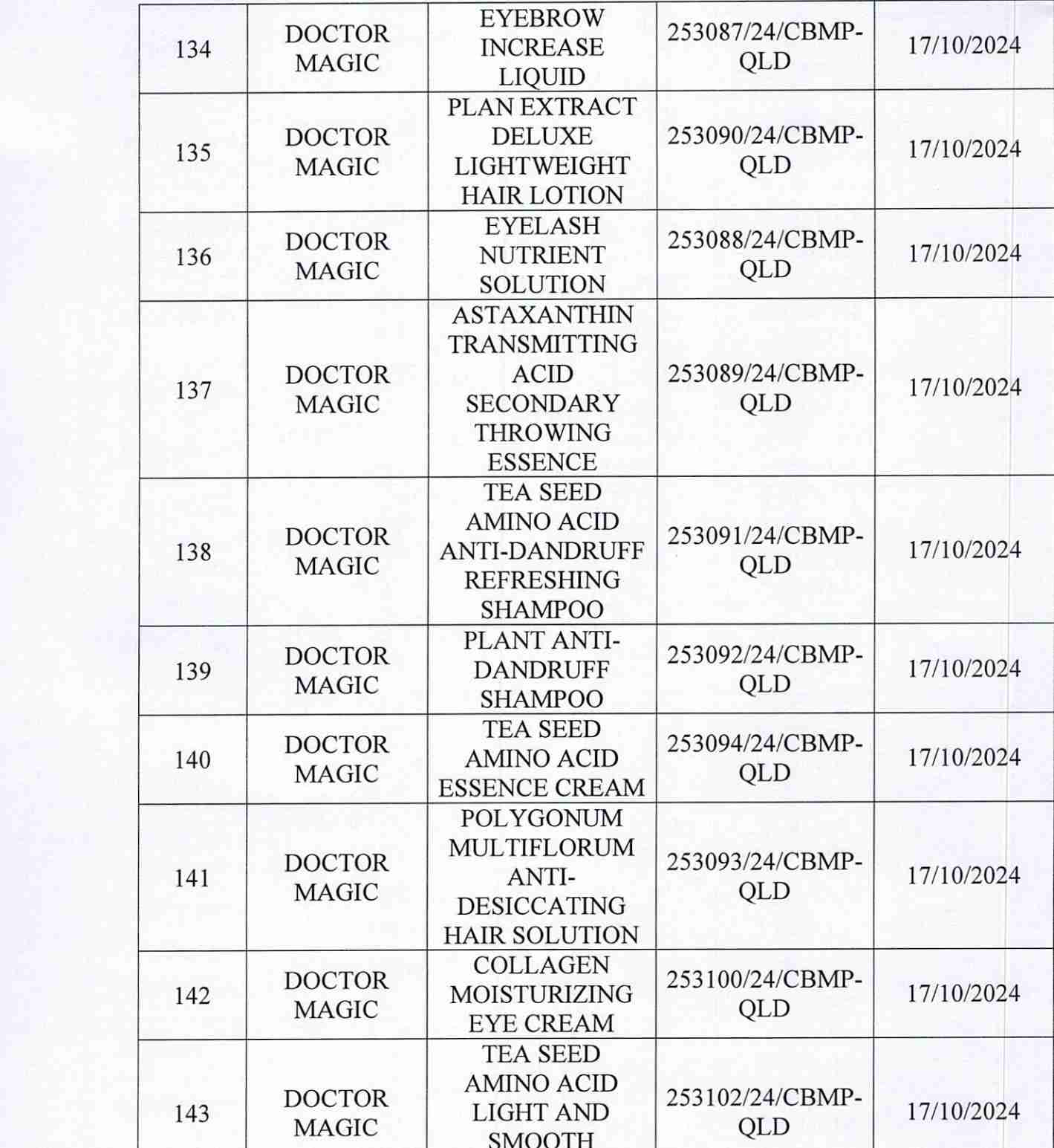
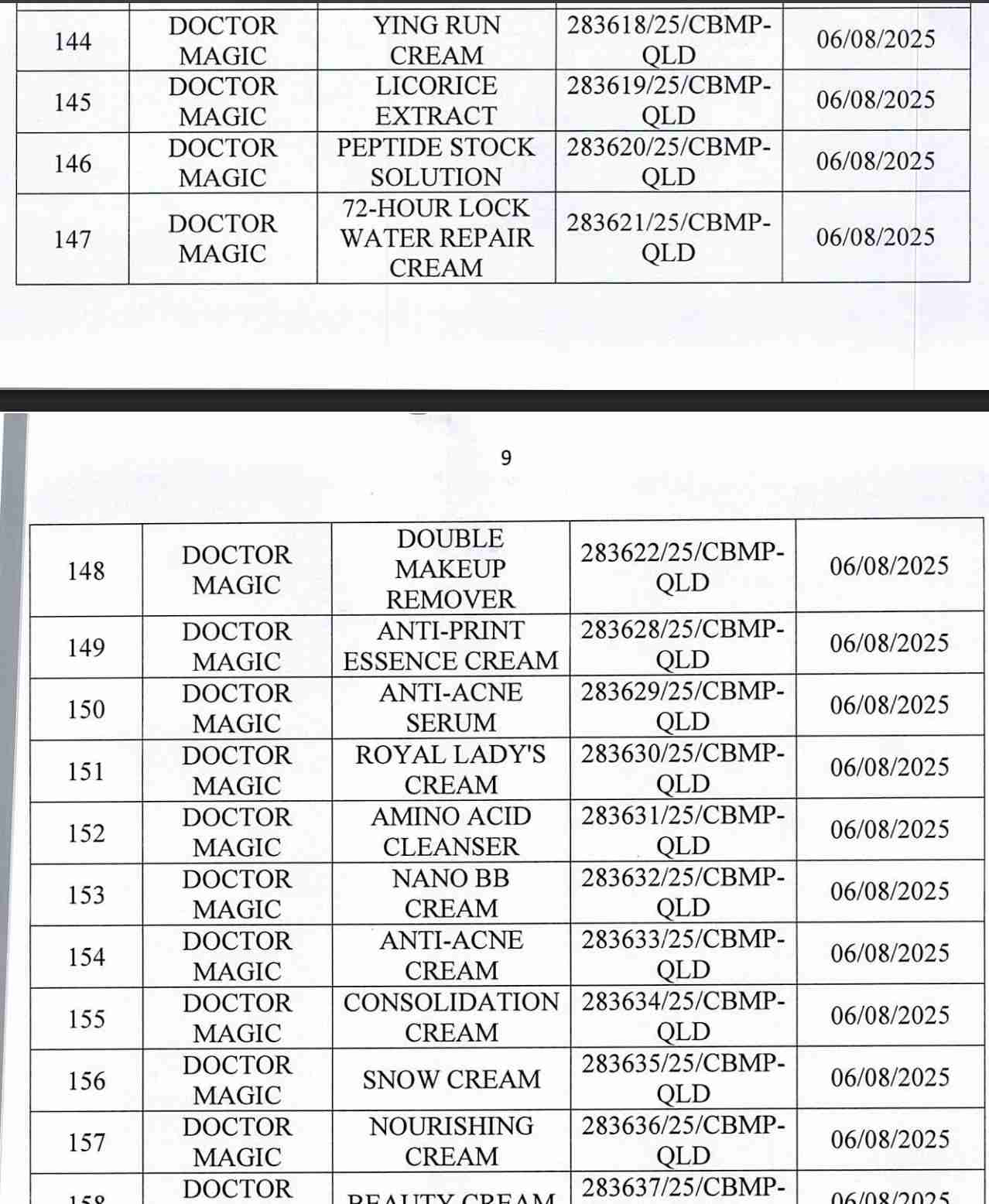
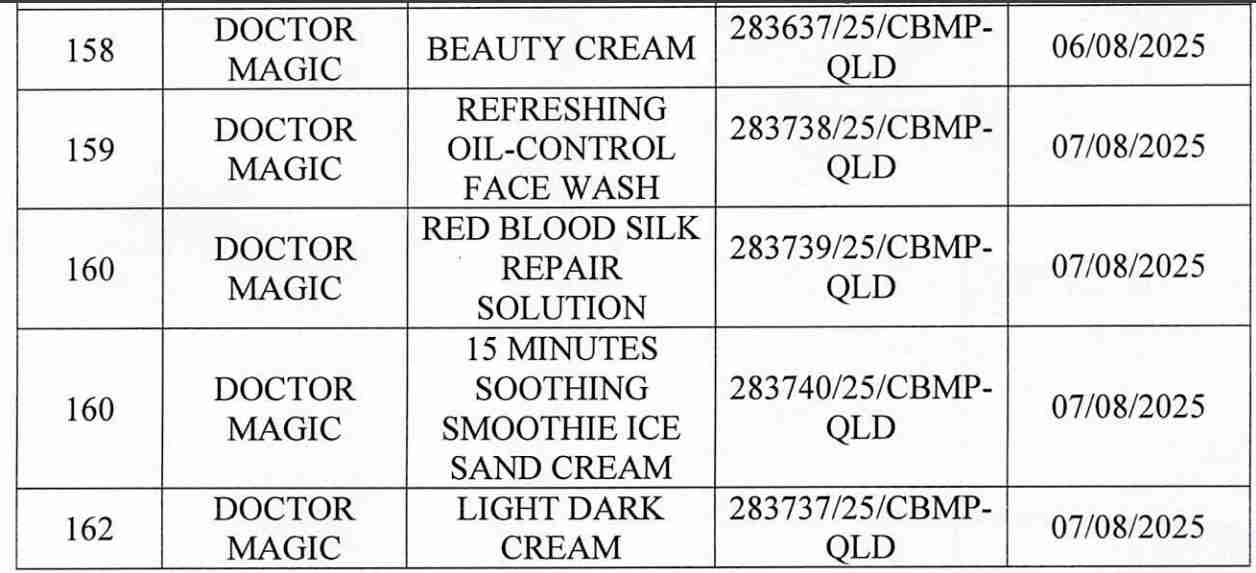
For 162 products that have been granted a cosmetic product declaration receipt number that the Company is responsible for launching on the market, it is recommended to provide a full report on the corresponding product information records.
Request the Company to send the full report to the Department of Drug Administration before November 21, 2025.
On the same day, the Department also sent an official dispatch to the Departments of Health of provinces and cities regarding the provision of information on inspection, examination and handling of administrative violations.
Departments of Health of provinces and cities: Conduct reviews and report inspection and examination results; report on handling administrative violations (if any) related to compliance with legal regulations on business, circulation and use of cosmetic products with the brands "DOCTOR MAGIC", "MAIKA BEAUTY", "MK" issued by MK Skin Import-Export Production and Trading Services Company Limited.
For the Ho Chi Minh City Department of Health: Conduct an inspection of MK Skincare Import-Export Production and Trading Service Company Limited in the area; assess compliance with legal regulations on cosmetic management in cosmetic import, trading and advertising activities; handle and punish violations in accordance with current regulations (if violations are detected);
Dr. Ta Manh Hung, Deputy Director of the Department of Drug Administration (Ministry of Health) also said: Regarding the implementation of Sampling, testing and strengthening quality supervision of cosmetic products of the brands "DOCTOR MAGIC", "MAIKA BEAUTY", "MK" of MK Skincare Company, the Department of Drug Administration recommends that the Department of Health of provinces and cities; the Central Institute for Drug Testing; the Ho Chi Minh City Institute for Drug Testing: Direct the affiliated Pharmaceutical - Cosmetic Testing Center; Organize the collection of cosmetic samples for circulation in the area; Focus on collecting samples of products under the brands "DOCTOR MAGIC", "MAKA BEAUT", "MKK" imported and marketed by MKcare Company.
" priorize the collection of samples for products in the high-risk group, including: Skin whitening products; sunscreens; products with labels or instructions for pregnant women or children," Dr. Hung emphasized.
For the Central Institute for Drug Testing and the Ho Chi Minh City Institute for Drug Testing: Increase sampling of cosmetic products under the brands "DOCTOR MAGIC", "MAIKA BEAUTY", "MK" of MK Skincare Company nationwide, especially in: Major cities, theme stores, E-commerce platforms. Providing professional and technical support for the Testing Centers of provinces/cities when the samples are suspected of containing banned substances or ingredients exceeding the prescribed limit; When it is necessary to compare test methods or perform specialized analysis techniques.