Tam Anh General Hospital system is the first and only unit in addition to the US participating in clinical research drugs for cancer treatment right from the stage 2a as the main research point. In phase 1 study in the US, RBS2418 oral immunity recorded for safety and tolerance. In phase 2A, research continues to evaluate the safety and potential of the drug.
RBS2418 was developed by riboscience Biofarmic Company (USA), with the participation of experts from Stanford University (USA). VISTA-1 was approved by the US Food and Drug Administration (FDA) in September 2024 and licensed by the Vietnamese Ministry of Health in December 2024.

Dr. Vu Huu Khiem, Head of the Oncology Department, Tam Anh Hanoi General Hospital, the main investigator, said that after 3 months, the hospital has received and screened hundreds of patients, of which 8 people were treated according to the VISTA-1 research regimen. The process of closely monitoring and recording no significant unusual symptoms related to the study drug.
For people with advanced stage colorectal cancer who no longer meet current treatment regimens, each advance in the extra life span during the study is very meaningful. Currently, we and the patient have entered the 4th month of implementing this study, Dr. Khiem shared.

Mr. C, 75 years old, with stage 3 colorectal cancer since 2023, has undergone chemotherapy, surgery, and treatment for lung metastasis using high- frequency wave burning and targeted drugs but had to stop because of side effects. When the VISTA-1 study started in Vietnam, he registered to participate and passed the screening at Tam Anh Hanoi General Hospital, becoming one of the few elderly patients eligible to participate.
"The medicine is oral, so I can use it at home, no need to be hospitalized, it is very convenient for the elderly and people with difficulty traveling like me," said Mr. C. After 1 month of treatment in the study, he was still living normally, not recording any significant signs of abnormality.
According to Dr. Khiem, initial feedback from patients is an important observation in the monitoring process, supporting the assessment of the safety and effectiveness of RBS24 18. Patients will continue to be monitored and evaluated at the expected time points of week 6, 12 and 20, through computer-based assessment scans and testing of biological indicators of the questionnaire to assess quality of life.
Prof. Dr. Jeffrey S. Glenn, Director of the Institute of microbiology & Control Stanford (USA), Head of the VISTA-1 research team, said he was very impressed with the progress of patient admission in Vietnam in the past 3 months.
Doctors at Tam Anh General Hospital, Vietnam have high expertise, experience in cancer treatment and have a deep understanding of international clinical research processes. We highly appreciate the patient selection and management process, as well as the quality of the laboratory and research infrastructure of Tam Anh General Hospital, especially the good coordination and efficiency between the research team in the US and Vietnam, Professor Jeffrey affirmed.
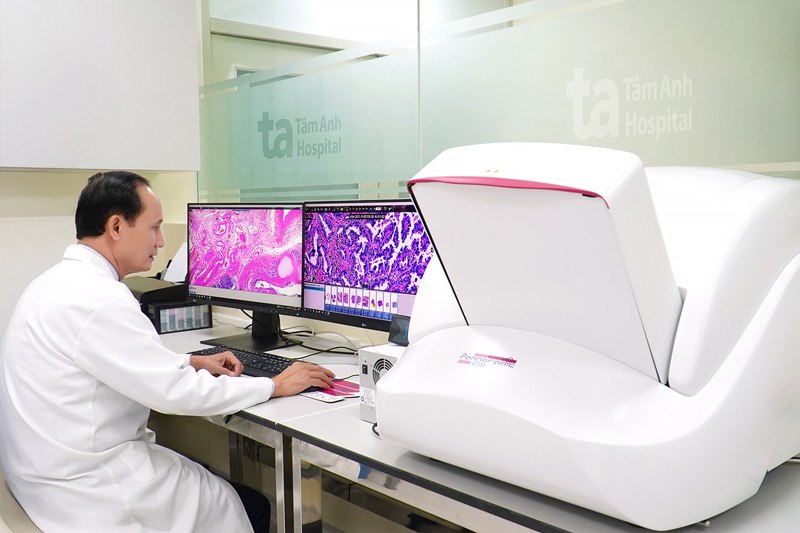
To prepare for the VISTA-1 research, Tam Anh General Hospital System and Tam Anh Research Institute (TAMRI) have invested heavily to develop a Labo center that meets ISO 15189 standards for all fields such as criminology, biochemistry, microbiology, and mobile biology. The Labo room is equipped with many modern specialized equipment for research, supporting the assessment of the level of expression of two biological markers ENPP1 and cGAS in cancer cells and tissue.
This is the first time these technologies have been deployed in the clinical research system in Vietnam. Tam Anh General Hospital is also the first unit in Vietnam to transfer testing techniques for the two new biological markers ENPP1 and cGAS, thereby qualifying to participate in the VISTA-1 research.

According to Dr. Phuong Le Tri, Executive Director of Tam Anh Research Institute (TAMRI), previously, Vietnamese cancer patients had little access to new drug regimens, so they had to go abroad for treatment. Now, thanks to international research such as VISTA-1, they can use new drugs right in Vietnam.
Vietnamese scientists and doctors have the opportunity to participate early in the world's leading modern, professional and advanced inventive drugs research process.
VISTA-1 research continues to recruit patients
The VISTA-1 study is still recruiting patients in Vietnam and the US. Participants are regularly monitored for their health, supported with research costs, travel expenses and 24/7 consultation by a team of experts.
The Oncology Department of Tam Anh General Hospital in Hanoi and Ho Chi Minh City opened a clinic "VISTA-1 Research Consulting", providing free consultation and checking participation conditions. If you or your loved one want to learn more about the clinical research of the drug RBS2418, please contact: cskh@tah Hospital.vn











